Source: FDA, National Drug Code (US) Revision Year: 2020
Omegaven (fish oil triglycerides) is a sterile, nonpyrogenic, white, homogenous emulsion for intravenous infusion as a supply of calories in patients with PNAC. Each mL of Omegaven contains 0.1 g of fish oil, 0.012 g egg phospholipids, 0.025 g glycerin, 0.15 to 0.3 mg dl-alpha-tocopherol, 0.3 mg sodium oleate, water for injection, and sodium hydroxide for pH adjustment (pH 6 to 9). The phosphate content is 0.015 mmol/mL.
The fish oil included in Omegaven is a triglyceride mixture consisting of esters of long-chain saturated fatty acids and unsaturated fatty acids with the following structure:
where 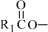
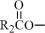
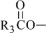
Omegaven 5 g/50 mL contains 5 grams of fish oil and 0.6 g egg phospholipids, 1.25 g glycerin, 7.5 to 15 mg dl-alpha-tocopherol, 0.015 g sodium oleate, water for injection, and sodium hydroxide for pH adjustment (pH 6 to 9) packaged in a single-dose 50-mL glass bottle enclosed with a rubber stopper. The phosphate content of the drug product is 0.75 mmol.
The mean content of the two major fatty acid components in 50 mL are 1.0 g EPA (range: 0.6 to 1.5 g) and 0.96 g DHA (range: 0.7 to 1.7 g). Additionally, the mean content of linoleic acid, alpha-linolenic acid, and arachidonic acid per 50 mL are 0.16 g, 0.07 g, and 0.13 g, respectively.
Omegaven 10 g/100 mL contains 10 grams of fish oil and 1.2 g egg phospholipids, 2.5 g glycerin, 15 to 30 mg dl-alpha-tocopherol, 0.03 g sodium oleate, water for injection, and sodium hydroxide for pH adjustment (pH 6 to 9) packaged in a single-dose 100-mL glass bottle enclosed with rubber stopper. The phosphate content of the drug product is 1.5 mmol. The mean content of the two major fatty acid components in 100 mL are 2.0 g EPA (range: 1.2 to 3.0 g) and 1.9 g DHA (range: 1.3 to 3.3 g). Additionally, the mean content of linoleic acid, alpha-linolenic acid, and arachidonic acid per 100 mL are 0.31g, 0.13 g, and 0.25 g; respectively.
The total energy content of Omegaven is 112 kcal/100 mL (1.12 kcal/mL), including lipids, phospholipids, and glycerol.
Omegaven has an osmolality of approximately 342 mOsm/kg water (which represents an osmolarity of 273 mOsm/L).
Omegaven contains no more than 25 mcg/L of aluminum.
| Dosage Forms and Strengths |
|---|
|
Injectable Emulsion: 5 g/50 mL and 10 g/100 mL (0.1 g/mL) sterile, white, homogenous emulsion in a 50-mL and 100-mL single-dose bottle. |
| How Supplied | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Omegaven (fish oil triglycerides) injectable emulsion, 5 g/50 mL and 10 g/100 mL (0.1 g/mL) is a white, homogenous, sterile emulsion supplied as follows:
The stopper used as the bottle closure is not made with natural rubber latex, PVC, or DEHP. Manufactured by: Graz, Austria |
| Drug | Countries | |
|---|---|---|
| OMEGAVEN | Austria, Brazil, Estonia, France, Hong Kong, Croatia, Israel, Lithuania, New Zealand, Poland, Romania, Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.