Cryopyrin associated periodic syndromes (CAPS)
Active Ingredient: Canacinumab
Indication for Canacinumab
Canakinumab is indicated for the treatment of cryopyrin-associated periodic syndromes (CAPS) including:
- Muckle-Wells syndrome (MWS),
- Neonatal-onset multisystem inflammatory disease (NOMID)/chronic infantile neurological, cutaneous, articular syndrome (CINCA),
- Severe forms of familial cold autoinflammatory syndrome (FCAS)/familial cold urticaria (FCU) presenting with signs and symptoms beyond cold-induced urticarial skin rash.
For this indication, competent medicine agencies globally authorize below treatments:
2-4 mg/kg once every 8 weeks
Route of admnistration
Subcutaneous
Defined daily dose
2 - 4 mg per kg of body weight
Dosage regimen
From 2 To 4 mg per kg of body weight once every 56 day(s)
Detailed description
Cryopyrin associated periodic syndromes (CAPS): Adults, adolescents and children aged 2 years and older
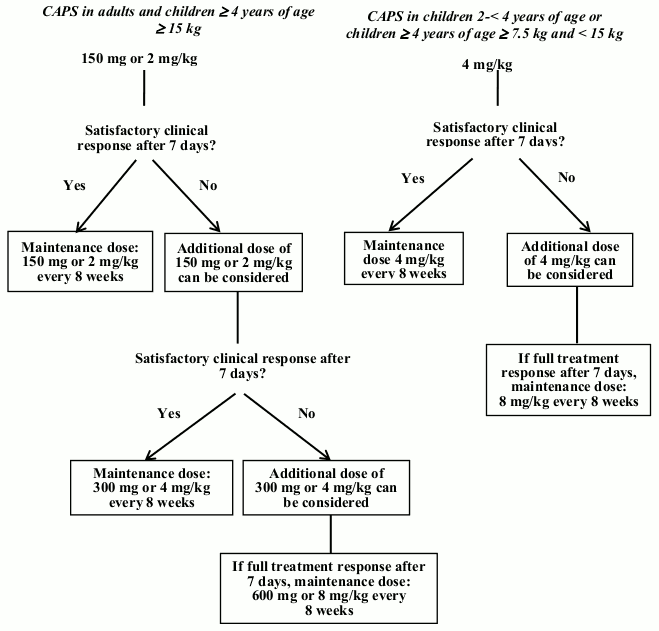
The recommended starting dose of canakinumab for cryopyrin associated periodic syndromes (CAPS) patients is:
Adults, adolescents and children ≥4 years of age:
- 150 mg for patients with body weight >40 kg
- 2 mg/kg for patients with body weight ≥15 kg and ≤40 kg
- 4 mg/kg for patients with body weight ≥7.5 kg and <15 kg
Children 2 to <4 years of age:
- 4 mg/kg for patients with body weight ≥7.5 kg
This is administered every eight weeks as a single dose via subcutaneous injection.
For patients with a starting dose of 150 mg or 2 mg/kg, if a satisfactory clinical response (resolution of rash and other generalised inflammatory symptoms) has not been achieved 7 days after treatment start, a second dose of canakinumab at 150 mg or 2 mg/kg can be considered. If a full treatment response is subsequently achieved, the intensified dosing regimen of 300 mg or 4 mg/kg every 8 weeks should be maintained. If a satisfactory clinical response has not been achieved 7 days after this increased dose, a third dose of canakinumab at 300 mg or 4 mg/kg can be considered. If a full treatment response is subsequently achieved, maintaining the intensified dosing regimen of 600 mg or 8 mg/kg every 8 weeks should be considered, based on individual clinical judgement.
For patients with a starting dose of 4 mg/kg, if a satisfactory clinical response has not been achieved 7 days after treatment start, a second dose of canakinumab 4 mg/kg can be considered. If a full treatment response is subsequently achieved, maintaining the intensified dosing regimen of 8 mg/kg every 8 weeks should be considered, based on individual clinical judgement.
Clinical experience with dosing at intervals of less than 4 weeks or at doses above 600 mg or 8 mg/kg is limited.
Dosage considerations
The following are suitable injection sites: upper thigh, abdomen, upper arm or buttocks. It is recommended to select a different injection site each time the product is injected to avoid soreness. Broken skin and areas which are bruised or covered by a rash should be avoided. Injection into scar tissue should be avoided as this may result in insufficient exposure to canakinumab.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.