Obstructive hypertrophic cardiomyopathy
Active Ingredient: Mavacamten
Indication for Mavacamten
Mavacamten is indicated for the treatment of symptomatic (New York Heart Association, NYHA, class II-III) obstructive hypertrophic cardiomyopathy (oHCM) in adult patients.
For this indication, competent medicine agencies globally authorize below treatments:
2.5 mg, 5 mg, 10 mg or 15 mg once daily
For:
Dosage regimens
Oral, between 2.5 milligrams mavacamten and 15 milligrams mavacamten, once daily.
Detailed description
The dose range is 2.5 mg to 15 mg (either 2.5 mg, 5 mg, 10 mg or 15 mg). The bioequivalence between strengths has not been confirmed in a bioequivalence study in humans.
CYP2C19 poor metaboliser phenotype
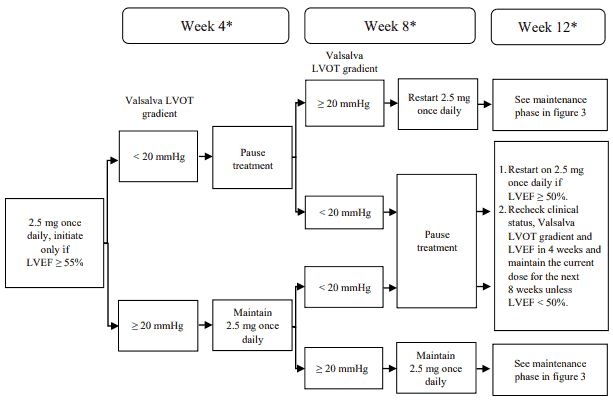
The recommended starting dose is 2.5 mg orally once daily. The maximum dose is 5 mg once daily. The patient should be assessed for early clinical response by left ventricular outflow tract (LVOT) gradient with Valsalva manoeuvre 4 and 8 weeks after treatment initiation (see figure 1).
CYP2C19 intermediate, normal, rapid and ultra-rapid metaboliser phenotype
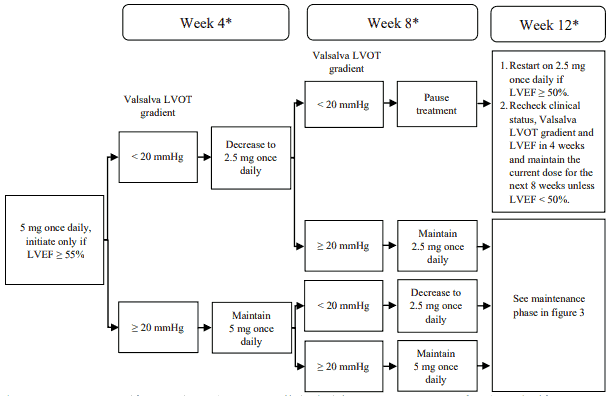
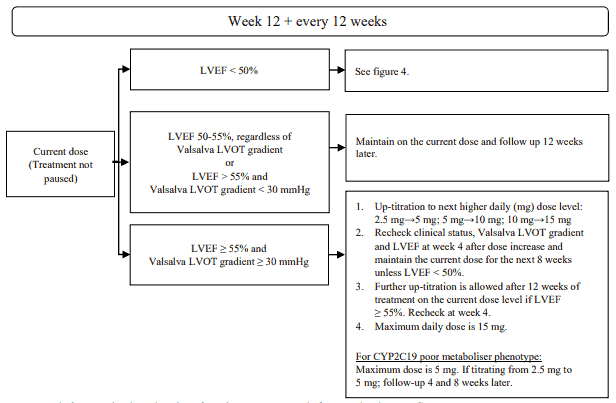
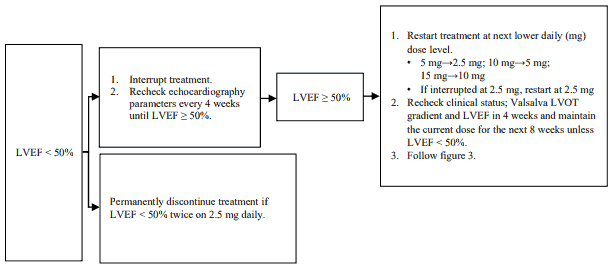
The recommended starting dose is 5 mg orally once daily. The maximum dose is 15 mg once daily. The patient should be assessed for early clinical response by LVOT gradient with Valsalva manoeuvre 4 and 8 weeks after treatment initiation (see figure 2). Once an individualised maintenance dose is achieved, patients should be assessed every 12 weeks (see figure 3). If at any visit the patient's LVEF is <50%, the treatment should be interrupted for 4 weeks and until LVEF returns to ≥50% (see figure 4).
In patients experiencing an intercurrent illness such as serious infection or arrhythmia (including atrial fibrillation or other uncontrolled tachyarrhythmia) which may impair systolic function, LVEF assessment is recommended, and dose increases are not recommended until intercurrent illness is resolved.
Consideration should be given to discontinue treatment in patients who have shown no response (e.g., no improvement in symptoms, quality of life, exercise capacity, LVOT gradient) after 4-6 months on the maximum tolerated dose.
Figure 1. Treatment initiation in CYP2C19 poor metaboliser phenotype:
* Interrupt treatment if LVEF is <50% at any clinical visit; restart treatment after 4 weeks if
LVEF ≥50% (see figure 4).
LVEF = left ventricular ejection fraction; LVOT = left ventricular outflow tract
Figure 2. Treatment initiation in CYP2C19 intermediate, normal, rapid and ultra-rapid metaboliser phenotype:
* Interrupt treatment if LVEF is <50% at any clinical visit; restart treatment after 4 weeks if
LVEF ≥50% (see figure 4).
LVEF = left ventricular ejection fraction; LVOT = left ventricular outflow tract
Figure 3. Maintenance phase:
LVEF = left ventricular ejection fraction; LVOT = left ventricular outflow tract
Figure 4. Treatment interruption at any clinic visit if LVEF <50%:
LVEF = left ventricular ejection fraction; LVOT = left ventricular outflow tract
Missed or delayed doses
If a dose is missed, it should be taken as soon as possible, and the next scheduled dose should be taken at the usual time the following day. Two doses should not be taken on the same day.
Dosage considerations
Treatment should be taken once daily with or without meals at about the same time each day.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.