Crow’s feet lines seen at maximum smile
Active Ingredient: Botulinum toxin type A
Indication for Botulinum toxin type A
For this indication, competent medicine agencies globally authorize below treatments:
24 Units as 4 Units injections across 6 sites
For:
Dosage regimens
Subcutaneous, 24 international units botulinum toxin type A, once every 3 months.
Detailed description
Recommended needle: Sterile 30 gauge needle.
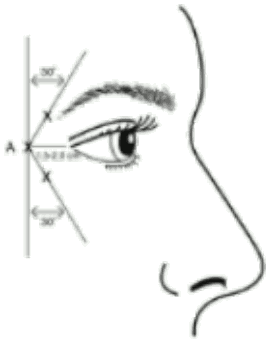
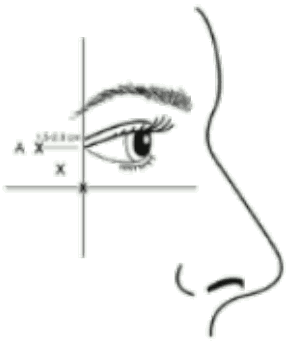
Administration guidance: Injections should be given with the needle tip bevel up and oriented away from the eye. The first injection (A) should be made approximately 1.5 to 2.0 cm temporal to the lateral canthus and just temporal to the orbital rim. If the lines in the crow’s feet region are above and below the lateral canthus, inject as shown in Figure 1. Alternatively, if the lines in the crow’s feet region are primarily below the lateral canthus, inject as shown in Figure 2.
In order to reduce the risk of eyelid ptosis, injections should be made temporal to the orbital rim, thereby maintaining a safe distance from the muscle controlling eyelid elevation.
Figure 1:
Figure 2:
Care should be taken to ensure that botulinum toxin type A is not injected into a blood vessel when it is injected in the crow’s feet lines seen at maximum smile.
Recommended dose: A volume of 0.1 ml (4 Units) is administered in each of the 3 injection sites per side (total of 6 injection sites) in the lateral orbicularis oculi muscle, for a total dose of 24 Units in a total volume of 0.6 ml (12 Units per side).
For simultaneous treatment with glabellar lines seen at maximum frown, the dose is 24 Units for crow’s feet lines seen at maximum smile and 20 Units for glabellar lines for a total dose of 44 Units in a total volume of 1.1 ml.
Maximum dose: In order to reduce the risk of eyelid ptosis, the maximum dose of 4 Units for each injection site as well as the number of injection sites should not be exceeded.
Additional information: Treatment intervals should not be more frequent than every 3 months.
The efficacy and safety of repeat injections of botulinum toxin type A for the treatment of crow’s feet lines beyond 12 months has not been evaluated.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.