Multiple myeloma
Active Ingredient: Ixazomib
Indication for Ixazomib
Ixazomib in combination with lenalidomide and dexamethasone is indicated for the treatment of adult patients with multiple myeloma who have received at least one prior therapy.
For this indication, competent medicine agencies globally authorize below treatments:
4 mg once a week on days 1, 8, and 15 of a 28-day treatment cycle
Route of admnistration
Oral
Defined daily dose
4 - 4 mg
Dosage regimen
From 4 To 4 mg once every 7 day(s)
Detailed description
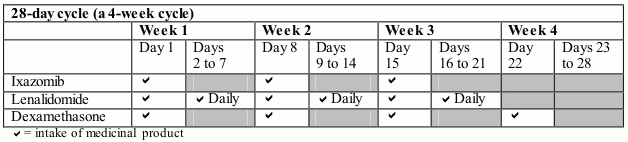
The recommended starting dose of ixazomib is 4 mg administered orally once a week on Days 1, 8, and 15 of a 28-day treatment cycle.
The recommended starting dose of lenalidomide is 25 mg administered daily on Days 1 to 21 of a 28-day treatment cycle.
The recommended starting dose of dexamethasone is 40 mg administered on Days 1, 8, 15, and 22 of a 28-day treatment cycle.
Dosing schedule. Ixazomib taken with lenalidomide and dexamethasone:
Prior to initiating a new cycle of therapy:
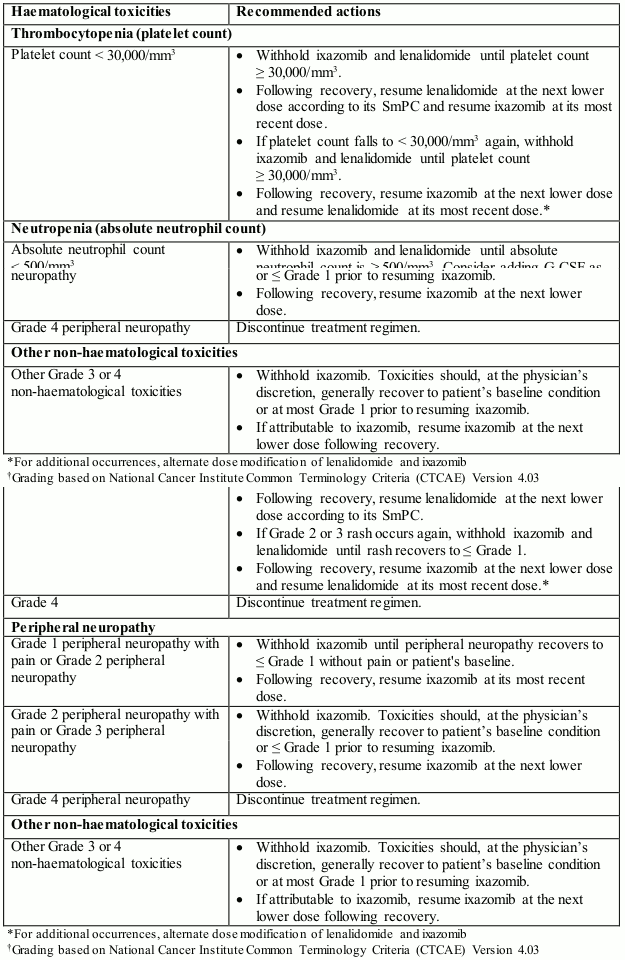
- Absolute neutrophil count should be ≥1,000/mm³
- Platelet count should be ≥75,000/mm³
- Non-haematologic toxicities should, at the physician's discretion, generally be recovered to patient's baseline condition or ≤ Grade 1
Treatment should be continued until disease progression or unacceptable toxicity. Treatment with ixazomib in combination with lenalidomide and dexamethasone for longer than 24 cycles should be based on an individual benefit risk assessment, as the data on the tolerability and toxicity beyond 24 cycles are limited.
Delayed or missed doses
In the event that a ixazomib dose is delayed or missed, the dose should be taken only if the next scheduled dose is ≥72 hours away. A missed dose should not be taken within 72 hours of the next scheduled dose. A double dose should not be taken to make up for a missed dose.
If a patient vomits after taking a dose, the patient should not repeat the dose but should resume dosing at the time of the next scheduled dose.
Dose modifications
The ixazomib dose reduction steps are presented in Table 1 and the dose modification guidelines are provided in Table 2.
Table 1. Ixazomib dose reduction steps:
| Recommended starting dose* | First reduction to | Second reduction to | Discontinue |
| 4 mg | 3 mg | 2.3 mg |
* Recommended reduced dose of 3 mg in the presence of moderate or severe hepatic impairment, severe renal impairment or end-stage renal disease (ESRD) requiring dialysis.
An alternating dose modification approach is recommended for ixazomib and lenalidomide for overlapping toxicities of thrombocytopenia, neutropenia and rash. For these toxicities, the first dose modification step is to withhold/reduce lenalidomide.
Table 2. Dose modifications guidelines for ixazomib in combination with lenalidomide and dexamethasone:
Concomitant medicinal products
Antiviral prophylaxis should be considered in patients being treated with ixazomib to decrease the risk of herpes zoster reactivation. Patients included in studies with ixazomib who received antiviral prophylaxis had a lower incidence of herpes zoster infection compared to patients who did not receive prophylaxis.
Thromboprophylaxis is recommended in patients being treated with ixazomib in combination with lenalidomide and dexamethasone, and should be based on an assessment of the patient's underlying risks and clinical status.
Dosage considerations
Ixazomib should be taken at approximately the same time on days 1, 8, and 15 of each treatment cycle at least 1 hour before or at least 2 hours after food.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.