Metastatic melanoma with a BRAF V600 mutation
Active Ingredient: Cobimetinib
Indication for Cobimetinib
Cobimetinib is indicated for use in combination with vemurafenib for the treatment of adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.
For this indication, competent medicine agencies globally authorize below treatments:
60 mg once daily
Route of admnistration
Oral
Defined daily dose
60 - 60 mg
Dosage regimen
From 60 To 60 mg once every day for 21 day(s)
Detailed description
The recommended dose of cobimetinib is 60 mg once daily.
Cobimetinib is taken on a 28 day cycle. Each dose consists of three 20 mg tablets (60 mg) and should be taken once daily for 21 consecutive days (Days 1 to 21-treatment period); followed by a 7-day break (Days 22 to 28-treatment break). Each subsequent cobimetinib treatment cycle should start after the 7-day treatment break has elapsed.
Duration of treatment
Treatment with cobimetinib should continue until the patient no longer derives benefit or until the development of unacceptable toxicity (see Table 1 below).
Missed doses
If a dose is missed, it can be taken up to 12 hours prior to the next dose to maintain the once-daily regimen.
Vomiting
In case of vomiting after administration of cobimetinib, the patient should not take an additional dose on that day and treatment should be continued as prescribed the following day.
General dose modifications
The decision on whether to reduce the dose for either or both treatments should be based on the prescriber's assessment of individual patient safety or tolerability. Dose modification of cobimetinib is independent of vemurafenib dose modification.
If doses are omitted for toxicity, these doses should not be replaced. Once the dose has been reduced, it should not be increased at a later time.
Table 1 below gives general cobimetinib dose modification guidance.
Table 1. Recommended cobimetinib dose modifications:
| Grade (CTC-AE)* | Recommended cobimetinib dose |
|---|---|
| Grade 1 or Grade 2 (tolerable) | No dose reduction. Maintain cobimetinib at a dose of 60 mg once daily |
| Grade 2 (intolerable) or Grade 3/4 | |
| 1st Appearance | Interrupt treatment until Grade ≤ 1, restart treatment at 40 mg once daily |
| 2nd Appearance | Interrupt treatment until Grade ≤ 1, restart treatment at 20 mg once daily |
| 3rd Appearance | Consider permanent discontinuation |
* The intensity of clinical adverse events graded by the Common Terminology Criteria for Adverse Events v4.0 (CTC-AE)
Dose modification advice for haemorrhage
Grade 4 events or cerebral haemorrhage: Cobimetinib treatment should be interrupted. Cobimetinib treatment should be permanently discontinued for haemorrhage events attributed to cobimetinib.
Grade 3 events: cobimetinib treatment should be interrupted during evaluation to avoid any potential contribution to the event. There is no data on the effectiveness of cobimetinib dose modification for haemorrhage events. Clinical judgment should be applied when considering restarting cobimetinib treatment. Vemurafenib dosing can be continued when cobimetinib treatment is interrupted, if clinically indicated.
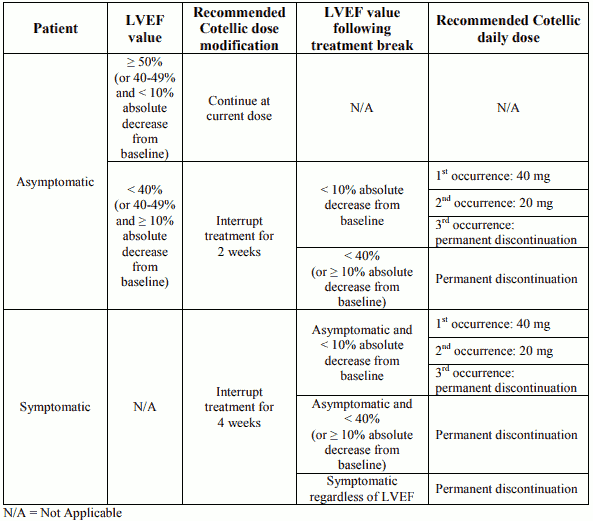
Dose modification advice for left ventricular dysfunction
Permanent discontinuation of cobimetinib treatment should be considered if cardiac symptoms are attributed to cobimetinib and do not improve after temporary interruption.
Table 2. Recommended dose modifications for cobimetinib in patients with left ventricular ejection fraction (LVEF) decrease from baseline:
Vemurafenib treatment can be continued when cobimetinib treatment is modified, if clinically indicated.
Dose modification advice for rhabdomyolysis and Creatine phosphokinase (CPK) elevations
Rhabdomyolysis or symptomatic CPK elevations
Cobimetinib treatment should be interrupted. If rhabdomyolysis or symptomatic CPK elevations do not improve within 4 weeks, cobimetinib treatment should be permanently discontinued. If severity is improved by at least one grade within 4 weeks, cobimetinib could be restarted at a dose reduced by 20 mg, if clinically indicated. Patients should be closely monitored. Vemurafenib dosing can be continued when cobimetinib treatment is modified.
Asymptomatic CPK elevations
Grade 4: Cobimetinib treatment should be interrupted. If CPK elevations do not improve to Grade ≤3 within 4 weeks following dose interruption, cobimetinib treatment should be permanently discontinued.
If CPK improves to Grade ≤3 within 4 weeks, cobimetinib could be restarted, if clinically indicated, at a dose reduced by 20 mg and the patient should be closely monitored. Vemurafenib dosing can be continued when cobimetinib treatment is modified.
Grade ≤3: After rhabdomyolysis has been ruled out, cobimetinib dosing does not need to be modified.
Dose modification advice for cobimetinib when used with vemurafenib
Liver laboratory abnormalities
For Grade 1 and 2 liver laboratory abnormalities, cobimetinib and vemurafenib should be continued at the prescribed dose.
Grade 3: Cobimetinib should be continued at the prescribed dose. The dose of vemurafenib may be reduced as clinically appropriate.
Grade 4: Cobimetinib treatment and vemurafenib treatment should be interrupted. If liver laboratory abnormalities improve to Grade ≤1 within 4 weeks, cobimetinib should be restarted at a dose reduced by 20 mg and vemurafenib at a clinically appropriate dose, per its SmPC.
Cobimetinib treatment and vemurafenib treatment should be discontinued if liver laboratory abnormalities do not resolve to Grade ≤1 within 4 weeks or if Grade 4 liver laboratory abnormalities recur after initial improvement.
Dosage considerations
It can be taken with or without food.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.