Pompe disease, late-onset
Active Ingredient: Cipaglucosidase alfa
Indication for Cipaglucosidase alfa
Cipaglucosidase alfa is a long-term enzyme replacement therapy used in combination with the enzyme stabiliser miglustat for the treatment of adults with late-onset Pompe disease (acid α-glucosidase [GAA] deficiency).
For this indication, competent medicine agencies globally authorize below treatments:
20 mg/kg of body weight every other week
For:
Dosage regimens
Intravenous, 20 milligrams cipaglucosidase alfa per kilogram of body weight, once every 2 weeks.
Detailed description
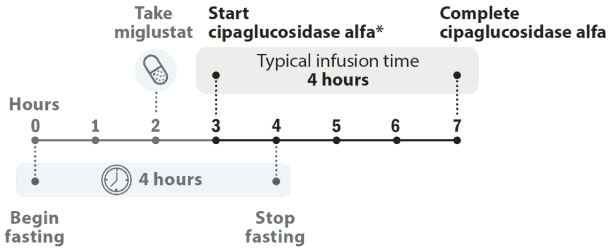
The recommended dose of cipaglucosidase alfa is 20 mg/kg of body weight every other week. The cipaglucosidase alfa infusion should start 1 hour after taking miglustat capsules. In the event of infusion delay, the start of infusion should not exceed 3 hours from taking miglustat.
Figure 1. Dose timeline:
* The cipaglucosidase alfa infusion should start 1 hour after taking miglustat capsules. In the event of infusion delay, the start of infusion should not exceed 3 hours from taking miglustat.
Patient response to treatment should be routinely evaluated based on a comprehensive evaluation of all clinical manifestations of the disease. In case of an insufficient response or intolerable safety risks, discontinuation of cipaglucosidase alfa in combination with miglustat treatment should be considered. Both medicinal products should either be continued or discontinued.
Switching patients from another enzyme replacement therapy (ERT)
If the patient is switching from another ERT to cipaglucosidase alfa in combination with miglustat therapy, the patient can be started with cipaglucosidase alfa-miglustat therapy at the next scheduled dosing time (i.e. approximately 2 weeks after the last ERT administration).
Patients who have switched from another ERT to cipaglucosidase alfa in combination with miglustat therapy should be advised to continue with any premedications used with the previous ERT therapy to minimise infusion-associated reactions (IARs). Depending on tolerability, premedication may be modified.
Missed dose
If the cipaglucosidase alfa infusion cannot be started within 3 hours of oral administration of miglustat, reschedule treatment of cipaglucosidase alfa and miglustat at least 24 hours after taking miglustat. If cipaglucosidase alfa and miglustat are both missed, treatment should occur as soon as possible.
Elderly
There is limited experience with the use of cipaglucosidase alfa in combination with miglustat therapy in patients above the age of 65 years old. There is no dose adjustment required in elderly patients.
Dosage considerations
Infusion of the 20 mg/kg dose is normally administered over the course of 4 hours if tolerated, 1 hour after taking miglustat capsules. Infusion should be administered in a stepwise manner. An initial cipaglucosidase alfa infusion rate of 1 mg/kg/hr is recommended. This infusion rate may be gradually increased by 2 mg/kg/hr approximately every 30 minutes if there are no signs of IARs until a maximum infusion rate of 7 mg/kg/hr is reached. The rate of infusion should be guided by the patient’s previous experience during infusion. The infusion rate may be slowed or temporarily stopped in the event of mild to moderate IARs. In the event of severe allergic, anaphylaxis, serious or severe IARs, the administration should immediately be discontinued, and appropriate medical treatment should be initiated.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.