Chronic migraine
Active Ingredient: Botulinum toxin type A
Indication for Botulinum toxin type A
Prophylaxis of headaches in adults with chronic migraine (headaches on at least 15 days per month of which at least 8 days are with migraine).
For this indication, competent medicine agencies globally authorize below treatments:
155-195 Units as 5 Units injections to 31 and up to 39 sites
For:
Dosage regimens
Intramuscular, between 155 international units botulinum toxin type A and 195 international units botulinum toxin type A, once every 12 weeks.
Detailed description
Recommended needle: Sterile 30 gauge, 0.5 inch needle.
A 1 inch needle may be needed in the neck region for patients with extremely thick neck muscles.
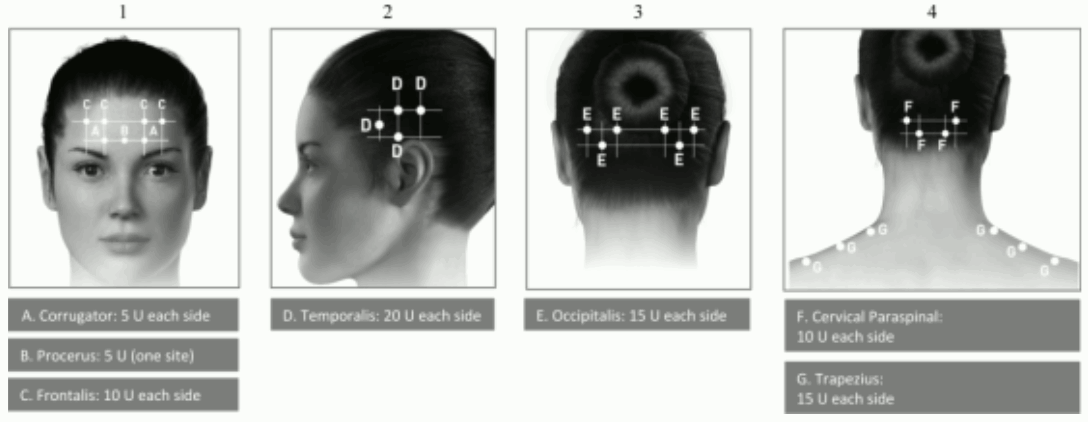
Administration guidance: Injections should be divided across 7 specific head/neck muscle areas as specified in the diagrams below. With the exception of the procerus muscle, which should be injected at 1 site (midline), all muscles should be injected bilaterally with half the number of injection sites administered to the left, and half to the right side of the head and neck.
The following diagrams indicate the injection sites:
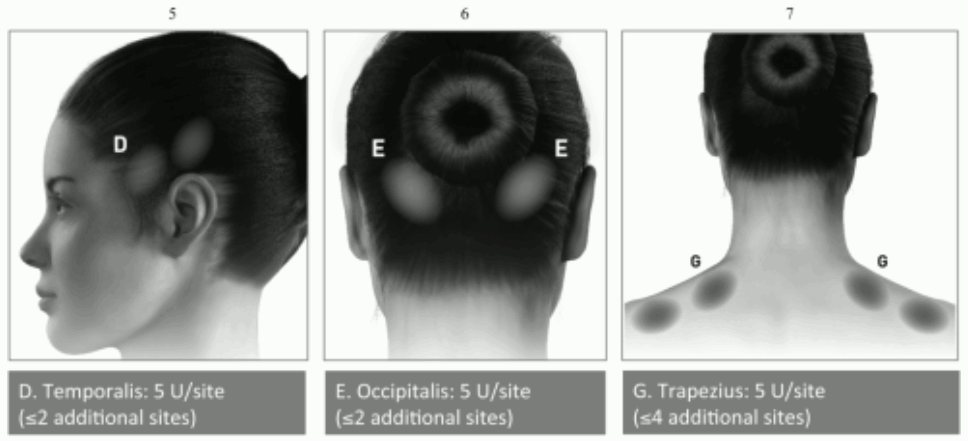
If there is a predominant pain location(s), additional injections to one or both sides may be administered in up to 3 specific muscle groups (occipitalis, temporalis and trapezius), up to the maximum dose per muscle as indicated in the table below.
The following diagrams indicate recommended muscle groups for optional additional injections:
Recommended dose: 155 Units to 195 Units administered intramuscularly as 0.1 ml (5 Units) injections to 31 and up to 39 sites.
| Recommended Dose | |
|---|---|
| Head/Neck Area | Total Dosage (number of sites*) |
| Corrugator** | 10 Units (2 sites) |
| Procerus | 5 Units (1 site) |
| Frontalis** | 20 Units (4 sites) |
| Temporalis** | 40 Units (8 sites) up to 50 Units (up to 10 sites) |
| Occipitalis** | 30 Units (6 sites) up to 40 Units (up to 8 sites) |
| Cervical Paraspinal Muscle Group** | 20 Units (4 sites) |
| Trapezius** | 30 Units (6 sites) up to 50 Units (up to 10 sites) |
| Total Dose Range: | 155 Units to 195 Units 31 to 39 sites |
* 1 IM injection site = 0.1 ml = 5 Units botulinum toxin type A
** Dose distributed bilaterally
Additional information: The recommended re-treatment schedule is every 12 weeks.
Dosage considerations
The maximum cumulative dose should not exceed 400 Units in a 12-week interval.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.