BRIVIACT Film-coated tablet Ref.[6625] Active ingredients: Brivaracetam
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: UCB Pharma S.A., Allée de la Recherche 60, B-1070 Bruxelles, Belgium
Pharmacodynamic properties
Pharmacotherapeutic group: antiepileptics, other antiepileptics
ATC code: N03AX23
Mechanism of action
Brivaracetam displays a high and selective affinity for synaptic vesicle protein 2A (SV2A), a transmembrane glycoprotein found at presynaptic level in neurons and in endocrine cells. Although the exact role of this protein remains to be elucidated it has been shown to modulate exocytosis of neurotransmitters. Binding to SV2A is believed to be the primary mechanism for brivaracetam anticonvulsant activity.
Clinical efficacy and safety
The efficacy of brivaracetam for the adjunctive therapy of partial onset seizures (POS) was established in 3 randomized, double-blind, placebo-controlled, fixed-dose, multi-center clinical studies in subjects 16 years of age and older. The daily dose of brivaracetam ranged from 5 to 200 mg/day across these studies. All studies had an 8-week baseline period followed by a 12-week treatment period with no up-titration. 1,558 patients received study drug of which 1,099 received brivaracetam. Study enrollment criteria required that patients have uncontrolled POS despite treatment with either 1 or 2 concomitant AEDs. Patients were required to have at least 8 POS during the baseline period. The primary endpoints in the phase 3 studies were the percent reduction in POS frequency over placebo and the 50% responder rate based on 50% reduction in POS frequency from baseline.
The most commonly taken AEDs at the time of study entry were carbamazepine (40.6%), lamotrigine (25.2%), valproate (20.5%), oxcarbazepine (16.0%), topiramate (13.5%), phenytoin (10.2%) and levetiracetam (9.8%). The median baseline seizure frequency across the 3 studies was 9 seizures per 28 days. Patients had a mean duration of epilepsy of approximately 23 years.
The efficacy outcomes are summarized in Table 2. Overall, brivaracetam was efficacious for the adjunctive treatment of partial onset seizures in patients 16 years of age and older between 50 mg/day and 200 mg/day.
Table 2. Key Efficacy Outcomes for Partial Onset Seizure Frequency per 28 Days:
| Study | Placebo | Brivaracetam *Statistically significant (p-value) | ||
|---|---|---|---|---|
| 50 mg/day | 100 mg/day | 200 mg/day | ||
| Study N012531 | ||||
| n=96 | n=101 | |||
| 50% Responder rate | 16.7 | 32.7* (p=0.008) | ~ | ~ |
| Percent reduction over placebo (%) | NA | 22.0* (p=0.004) | ~ | ~ |
| Study N012521 | ||||
| n=100 | n=99 | n=100 | ||
| 50% Responder rate | 20.0 | 27.3 (p=0.372) | 36.02 (p=0.023) | ~ |
| Percent reduction over placebo (%) | NA | 9.2 (p=0.274) | 20.52 (p=0.010) | |
| Study N01358 | ||||
| n=259 | n=252 | n=249 | ||
| 50% Responder rate | 21,6 | ~ | 38.9* (p<0.001) | 37.8* (p<0.001) |
| Percent reduction over placebo (%) | NA | ~ | 22.8* (p<0.001) | 23.2* (p<0.001) |
n = randomised patients who received at least 1 dose of study medication
~ Dose not studied
* Statistically significant
1 Approximately 20% of the patients were on concomitant levetiracetam
2 The primary outcome for N01252 did not achieve statistical significance based on the sequential testing procedure. The 100 mg/day dose was nominally significant.
In clinical studies, a reduction in seizure frequency over placebo was higher with the dose of 100 mg/day than with 50 mg/day. Apart from dose-dependent increases in incidences of somnolence and fatigue, brivaracetam 50 mg/day and 100 mg/day had a similar safety profile including CNS-related AEs and with long-term use.
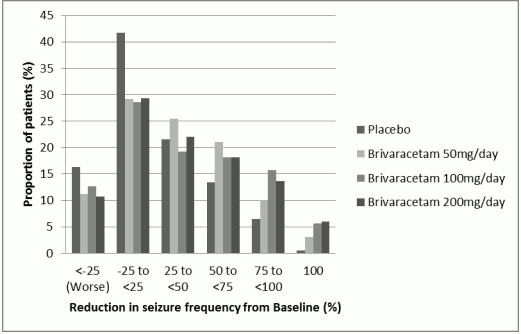
Figure 1 shows the percentage of patients (excluding patients with concomitant levetiracetam) by category of reduction from baseline in POS frequency per 28 days in all 3 studies. Patients with more than a 25% increase in POS are shown at left as "worse". Patients with an improvement in percent reduction in baseline POS frequency are shown in the 4 right-most categories. The percentages of patients with at least a 50% reduction in seizure frequency were 20.3%, 34.2%, 39.5%, and 37.8% for placebo, 50 mg/day, 100 mg/day, and 200 mg/day, respectively.
Figure 1. Proportion of patients by category of seizure response for brivaracetam and placebo over 12 weeks across all three double-blind pivotal clinical trials:
In a pooled analysis of the three pivotal trials, no differences in efficacy (measured as 50% responder rate) was observed within the dose range of 50 mg/day to 200 mg/day when brivaracetam is combined with inducing or non-inducing AEDs. In clinical studies 2.5% (4/161), 5.1% (17/332) and 4.0% (10/249) of the patients on brivaracetam 50 mg/day, 100 mg/day and 200 mg/day respectively became seizure free during the 12-week treatment period compared with 0.5% (2/418) on placebo.
Improvement in the median percent reduction in seizure frequency per 28 days has been observed in patients with type IC seizure (secondary generalized tonic-clonic seizures) at baseline treated with brivaracetam (66.6% (n=62), 61.2% (n=100) and 82.1% (n=75) of the patients on brivaracetam 50 mg/day, 100 mg/day and 200 mg/day respectively as compared to placebo 33.3% (n=115)).
The efficacy of brivaracetam in monotherapy has not been established. Brivaracetam is not recommended for use in monotherapy.
Treatment with levetiracetam
In two phase 3 randomised placebo-controlled studies, levetiracetam was administered as concomitant AED in about 20% of the patients. Although the number of subjects is limited, there was no observed benefit of brivaracetam versus placebo in patients taking levetiracetam concurrently which may reflect competition at the SV2A binding site. No additional safety or tolerability concerns were observed.
In a third study, a pre-specified analysis demonstrated efficacy over placebo for 100 mg/day and 200 mg/day in patients with prior exposure to levetiracetam. The lower efficacy observed in these patients compared to the leveticacetam-naïve patients was likely due to the higher number of prior AEDs used and higher baseline seizure frequency.
Elderly (65 years of age and above)
The three pivotal double-blind placebo-controlled clinical studies included 38 elderly patients aged between 65 and 80 years. Although data are limited, the efficacy was comparable to younger subjects.
Open label extension studies
Across all studies, 81.7% of the patients who completed randomized studies were enrolled in the long-term open-label extension studies. From entry into the randomized studies, 5.3% of the subjects exposed to brivaracetam for 6 months (n=1,500) were seizure free compared to 4.6% and 3.7% for subjects exposed for 12 months (n=1,188) and 24 months (n=847), respectively. However, as a high proportion of subjects (26%) discontinued from the open-label studies due to lack of efficacy, a selection bias may have occurred, as the subjects who stayed in the study responded better than those who have terminated prematurely.
In patients who were followed up in the open-label extension studies for up to 8 years, the safety profile was similar to that observed in the short-term, placebo-controlled studies.
Paediatric population
In children aged 2 years and older, partial onset seizures have a similar pathophysiology to those in adolescents and adults. Experience with epilepsy medicines suggests that the results of efficacy studies performed in adults can be extrapolated to children down to the age of 2 years provided the paediatric dose adaptations are established and safety has been demonstrated (see sections 5.2 and 4.8). Doses in patients from 2 years of age were defined by weight-based dose adaptations which have been established to achieve similar plasma concentrations to the ones observed in adults taking efficacious doses (section 5.2).
A long-term, uncontrolled, open-label safety study included children (from 1 month of age to less than 16 years) who continued treatment after completing the PK study (see section 5.2), children who continued treatment after completing the i.v. (intravenous) safety study and children directly enrolled into the safety study. Children who directly enrolled received a brivaracetam starting dose of 1 mg/kg/day and depending on response and tolerability, the dose was increased up to 5 mg/kg/day by doubling the dose at weekly intervals. No child received a dose greater than 200 mg/day. For children weighing 50 kg or greater the brivaracetam starting dose was 50 mg/day and depending on response and tolerability, the dose was increased up to a maximum of 200 mg/day by weekly increments of 50 mg/day.
From the pooled open-label safety and PK studies in adjunctive therapy, 186 children with POS in the age range of 1 month <16 years of age have received brivaracetam, of whom 149 have been treated for ≥3 months, 138 for ≥6 months, 123 for ≥12 months, 107 for ≥24 months, and 90 for ≥36 months.
The European Medicines Agency has deferred the obligation to submit the results of studies with brivaracetam in one or more subsets of the paediatric population in epilepsy with partial onset seizures (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Brivaracetam film-coated tablets, oral solution and solution for intravenous injection show the same AUC, while the maximum plasma concentration is slightly higher after intravenous administration. Brivaracetam exhibits linear and time-independent pharmacokinetics with low intra- and inter-subject variability, and features complete absorption, very low protein binding, renal excretion following extensive biotransformation, and pharmacologically inactive metabolites.
Absorption
Brivaracetam is rapidly and completely absorbed after oral administration and the absolute bioavailablity is approximately 100%. The median tmax for tablets taken without food is 1 hour (tmax range is 0.25 to 3 h).
Coadministration with a high-fat meal slowed down the absorption rate (median tmax 3 h) and decreased the maximum plasma concentration (37% lower) of brivaracetam, while the extent of absorption remained unchanged.
Distribution
Brivaracetam is weakly bound (≤20%) to plasma proteins. The volume of distribution is 0.5 L/kg, a value close to that of the total body water.
Due to its lipophylicity (Log P) brivaracetam has high cell membrane permeability.
Biotransformation
Brivaracetam is primarily metabolized by hydrolysis of the amide moiety to form the corresponding carboxylic acid (approximately 60% the elimination), and secondarily by hydroxylation on the propyl side chain (approximately 30% the elimination). The hydrolysis of the amide moiety leading to the carboxylic acid metabolite (34% of the dose in urine) is supported by hepatic and extra-hepatic amidase. In vitro, the hydroxylation of brivaracetam is mediated primarily by CYP2C19. Both metabolites, are further metabolised forming a common hydroxylated acid formed predominantly by hydroxylation of the propyl side chain on the carboxylic acid metabolite (mainly by CYP2C9). In vivo, in human subjects possessing ineffective mutations of CYP2C19, production of the hydroxy metabolite is decreased 10-fold while brivaracetam itself is increased by 22% or 42% in individuals with one or both mutated alleles. The three metabolites are not pharmacologically active.
Elimination
Brivaracetam is eliminated primarily by metabolism and by excretion in the urine. More than 95% of the dose, including metabolites, is excreted in the urine within 72 hours after intake. Less than 1% of the dose is excreted in faeces and less than 10% of brivaracetam is excreted unchanged in urine. The terminal plasma half-life (t1/2) is approximately 9 hours. The total plasma clearance in patients was estimated to 3.6 L/h.
Linearity
Pharmacokinetics is dose-proportional from 10 to at least 600 mg.
Interactions with medicinal products
Brivaracetam is cleared by multiple pathways including renal excretion, non-CYP-mediated hydrolysis and CYP-mediated oxidations. In vitro, brivaracetam is not a substrate of human P-glycoprotein (P-gp), multidrug resistance proteins (MRP) 1 and 2, and likely not organic anion transporter polypeptide 1B1 (OATP1B1) and OATP1B3. In vitro assays showed that brivaracetam disposition should not be significantly affected by CYP (eg. CYP1A, CYP2C8, CYP2C9, CYP2D6 and CYP3A4) inhibitors.
In vitro, brivaracetam was not an inhibitor of the CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2D6, CYP3A4, or the transporters P-gp, BCRP, BSEP MRP2, MATE-K, MATE-1, OATP1B1, OATP1B3, OAT1 and OCT1 at clinically relevant concentrations. In vitro, brivaracetam did not induce CYP1A2.
Pharmacokinetics in special patient groups
Elderly (65 years of age and above)
In a study in elderly subjects (65 to79 years old; with creatinine clearance 53 to 98 ml/min/1.73 m²) receiving brivaracetam 400 mg/day in bid administration, the plasma half-life of brivaracetam was 7.9 hours and 9.3 hours in the 65 to 75 and >75 years groups, respectively. The steady-state plasma clearance of brivaracetam was similar (0.76 ml/min/kg) to young healthy male subjects (0.83 ml/min/kg) (see section 4.2).
Renal impairment
A study in subjects with severe renal impairment (creatinine clearance <30 ml/min/1.73 m² and not requiring dialysis) revealed that the plasma AUC of brivaracetam was moderately increased (+21%) relative to healthy controls, while the AUC of the acid, hydroxy and hydroxyacid metabolites were increased 3-, 4-, and 21-fold, respectively. The renal clearance of these non active metabolites was decreased 10-fold. The hydroxyacid metabolite did not reveal any safety concerns in non clinical studies. Brivaracetam has not been studied in patients undergoing hemodialysis (see section 4.2).
Hepatic impairment
A pharmacokinetic study in subjects with hepatic cirrhosis (Child-Pugh classes A, B, and C) showed similar increases in exposure to brivaracetam irrespective of disease severity (50%, 57% and 59%), relative to matched healthy controls (see section 4.2).
Body weight
A 40% decrease in steady-state plasma concentration has been estimated across a body weight range from 46 kg to 115 kg. However, this is not considered to be a clinically relevant difference.
Gender
There are no clinically relevant differences in the pharmacokinetics of brivaracetam by gender.
Race
The pharmacokinetics of brivaracetam was not significantly affected by race (Caucasian, Asian) in a population pharmacokinetic modeling from epilepsy patients. The number of patients with other ethnic background was limited.
Pharmacokinetic/pharmacodynamics relationship
The EC50 (brivaracetam plasma concentration corresponding to 50% of the maximum effect) was estimated to be 0.57 mg/L. This plasma concentration is slightly above the median exposure obtained after brivaracetam doses of 50 mg/day. Further seizure frequency reduction is obtained by increasing the dose to 100 mg/day and reaches a plateau at 200 mg/day.
Paediatric population
In a pharmacokinetic study with a 3-week evaluation period and weekly fixed 3-step up-titration using the brivaracetam oral solution, 99 subjects aged 1 month to <16 years were evaluated. Brivaracetam was administered at weekly increasing doses of approximately 1 mg/kg/day, 2 mg/kg/day, and 4 mg/kg/day. All doses were adjusted by body weight, and did not exceed a maximum of 50 mg/day, 100 mg/day, and 200 mg/day. At the end of the evaluation period, subjects may have been eligible for entry into a long-term follow-up study continuing on their last received dose (see section 4.8). Plasma concentrations were shown to be dose-proportional in all age groups. Population pharmacokinetics modeling was performed based on sparse plasma concentration data collected in the 3-week PK study and the ongoing long-term follow-up study. 232 paediatric patients with epilepsy, aged 2 months to 17 years, were included in the analysis. The analysis indicated that doses of 5.0 (body weights 10-20 kg) and 4.0 mg/kg/day (body weights 20-50 kg) provide the same steady-state average plasma concentration as in adults receiving 200 mg/day. The estimated plasma clearance was 0.96 L/h, 1.61 L/h, 2.18 L/h and 3.19 L/h for children weighing 10 kg, 20 kg, 30 kg and 50 kg, respectively. In comparison, plasma clearance was estimated at 3.58 L/h in adult patients (70 kg body weight). Currently, no clinical data are available in neonates.
Preclinical safety data
In safety pharmacology studies, the predominant effects were CNS related (mainly transient CNS depression and decreased spontaneous locomotor activity) seen at multiples (greater than 50 fold) of the pharmacologically active dose of brivaracetam, 2 mg/kg. Learning and memory function were not affected.
Findings not observed in clinical studies, but seen in the repeated-dose toxicology dog studies at exposure similar to the clinical plasma AUC, were hepatotoxic effects (mainly porphyria). However, toxicological data accumulated on brivaracetam and on a structurally-related compound indicate that the dog liver changes have developed through mechanisms not relevant for humans. No adverse liver changes were seen in rats and monkeys following chronic administration of brivaracetam at 5- and 42-fold the clinical AUC exposure. In monkeys, CNS signs (prostrate, loss of balance, clumsy movements) occurred at 64 fold the clinical Cmax, these effects being less apparent over time.
Genotoxicity studies have not detected any mutagenic or clastogenic activity. Carcinogenicity studies did not indicate any oncogenic potential in rats, whereas increased incidences of hepatocellular tumors in male mice are considered to result of a non-genotoxic, mode of action linked to a phenobarbitone-like liver enzyme induction, which is a known rodent specific phenomenon.
Brivaracetam did not affect male or female fertility and has demonstrated no teratogenic potential in either rat or rabbit. Embryotoxicity was observed in rabbits at a maternal toxic dose of brivaracetam with an exposure level 8-fold the clinical AUC exposure at the maximum recommended dose. In rats, brivaracetam was shown to readily cross the placenta and to be excreted in milk of lactating rats with concentrations similar to maternal plasma levels.
Brivaracetam did not show any dependence potential in rats.
Juvenile animals studies
In juvenile rats, brivaracetam exposure levels 6- to 15-fold the clinical AUC exposure at the maximum recommended dose induced developmental adverse effects (i.e. mortality, clinical signs, decreased body weight and lower brain weight). There were no adverse effects on CNS function, neuropathological and brain histopathological examination. In juvenile dogs, the brivaracetam-induced changes at the exposure level 6-fold the clinical AUC were similar to those observed in adult animals. There were no adverse effects in any of the standard developmental or maturation endpoints.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.