Severe pain
Active Ingredient: Buprenorphine
Indication for Buprenorphine
Buprenorphine is indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.
For this indication, competent medicine agencies globally authorize below treatments:
35-70 mcg/hour
Route of admnistration
Percutaneous
Defined daily dose
35 - 70 ug
Dosage regimen
From 35 To 70 ug once every 4 day(s)
Detailed description
Important Dosage and Administration Information
Buprenorphine should be prescribed only by healthcare professionals who are knowledgeable in the use of potent opioids for the management of chronic pain.
Buprenorphine doses of 7.5, 10, 15, and 20 mcg/hour are only for use in patients who are opioid experienced and in whom tolerance to an opioid of comparable potency has been established. Patients who are opioid-experienced are those receiving, for one week or longer, daily opioid doses up to 80 mg/day of oral morphine or an equianalgesic dose of another opioid.
- Use the lowest effective dosage for the shortest duration consistent with individual patients treatment goals.
- Initiate the dosing regimen for each patient individually, taking into account the patient’s severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse.
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with buprenorphine.
- Instruct patients not to use buprenorphine if the pouch seal is broken or the patch is cut, damaged, or changed in any way and not to cut buprenorphine.
- Instruct patients to avoid exposing buprenorphine to external heat sources, hot water, or prolonged direct sunlight.
Buprenorphine is for transdermal use (on intact skin) only. Each buprenorphine patch is intended to be worn for 7 days.
Initial Dosage
Use of buprenorphine as the First Opioid Analgesic (opioid-naive patients)
Initiate treatment with buprenorphine with a 5 mcg/hour patch.
Conversion from Other Opioids to buprenorphine
Discontinue all other around-the-clock opioid drugs when buprenorphine therapy is initiated.
There is a potential for buprenorphine to precipitate withdrawal in patients who are already on opioids.
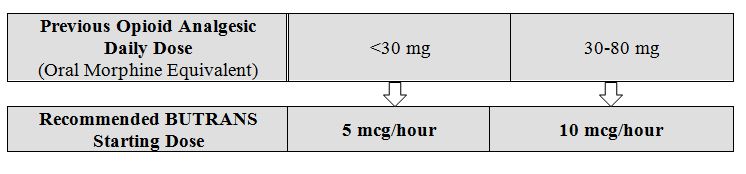
Prior Total Daily Dose of Opioid Less than 30 mg of Oral Morphine Equivalents per Day: Initiate treatment with buprenorphine 5 mcg/hour at the next dosing interval (see Table 1 below, middle column).
Prior Total Daily Dose of Opioid Between 30 mg to 80 mg of Oral Morphine Equivalents per Day:
Taper the patient’s current around-the-clock opioids for up to 7 days to no more than 30 mg of morphine or equivalent per day before beginning treatment with buprenorphine. Then initiate treatment with buprenorphine 10 mcg/hour at the next dosing interval (see Table 1 below, right column). Patients may use short-acting analgesics as needed until analgesic efficacy with buprenorphine is attained.
Prior Total Daily Dose of Opioid Greater than 80 mg of Oral Morphine Equivalents per Day: buprenorphine 20 mcg/hour may not provide adequate analgesia for patients requiring greater than 80 mg/day oral morphine equivalents. Consider the use of an alternate analgesic.
Table 1. Initial buprenorphine Dose:
Conversion from Methadone to buprenorphine:
Close monitoring is of particular importance when converting from methadone to other opioid agonists. The ratio between methadone and other opioid agonists may vary widely as a function of previous dose exposure. Methadone has a long half-life and can accumulate in the plasma.
Titration and Maintenance of Therapy
Individually titrate buprenorphine to a dose that provides adequate analgesia and minimizes adverse reactions. Continually reevaluate patients receiving buprenorphine to assess the maintenance of pain control and the relative incidence of adverse reactions, as well as monitoring for the development of addiction, abuse, or misuse. Frequent communication is important among the prescriber, other members of healthcare team, the patient, and the caregiver/family during periods of changing analgesic requirements, including initial titration. During chronic therapy, periodically reassess the continued need for opioid analgesics.
The minimum buprenorphine titration interval is 72 hours, based on the pharmacokinetic profile and time to reach steady state levels.
The maximum buprenorphine dose is 20 mcg/hour. Do not exceed a dose of one 20 mcg/hour buprenorphine system due to the risk of QTc interval prolongation. In a clinical trial, buprenorphine 40 mcg/hour (given as two buprenorphine 20 mcg/hour systems) resulted in prolongation of the QTc interval.
Patients who experience breakthrough pain may require dosage adjustment increase of buprenorphine, or may need rescue medication with an appropriate dose of an immediate-release analgesic. If the level of pain increases after dose stabilization, attempt to identify the source of increased pain before increasing the buprenorphine dose.
Because steady-state plasma concentrations are achieved within 72 hours, buprenorphine dosage may be adjusted every 3 days. Dose adjustments may be made in 5 mcg/hour, 7.5 mcg/hour, or 10 mcg/hour increments by using no more than two patches of the 5 mcg/hour, or 7.5 mcg/hour, or 10 mcg/hour system(s). The total dose from both patches should not exceed 20 mcg/hour. For the use of two patches, instruct patients to remove their current patch, and apply the two new patches at the same time, adjacent to one another at a different application site.
If unacceptable opioid-related adverse reactions are observed, consider reducing the dosage. Adjust the dosage to obtain an appropriate balance between the management of pain and opioid-related adverse reactions.
Safe Reduction or Discontinuation of buprenorphine
Do not abruptly discontinue buprenorphine in patients who may be physically dependent on opioids. Rapid discontinuation of opioid analgesics in patients who are physically dependent on opioids has resulted in serious withdrawal symptoms, uncontrolled pain, and suicide. Rapid discontinuation has also been associated with attempts to find other sources of opioid analgesics, which may be confused with drug-seeking for abuse. Patients may also attempt to treat their pain or withdrawal symptoms with illicit opioids, such as heroin, and other substances.
When a decision has been made to decrease the dose or discontinue therapy in an opioid-dependent patient taking buprenorphine, there are a variety of factors that should be considered, including the dose of buprenorphine the patient has been taking, the duration of treatment, the type of pain being treated, and the physical and psychological attributes of the patient. It is important to ensure ongoing care of the patient and to agree on an appropriate tapering schedule and follow-up plan so that patient and provider goals and expectations are clear and realistic. When opioid analgesics are being discontinued due to a suspected substance use disorder, evaluate and treat the patient, or refer for evaluation and treatment of the substance use disorder. Treatment should include evidence-based approaches, such as medication assisted treatment of opioid use disorder. Complex patients with comorbid pain and substance use disorders may benefit from referral to a specialist.
There are no standard opioid tapering schedules that are suitable for all patients. Good clinical practice dictates a patient-specific plan to taper the dose of the opioid gradually. For patients on buprenorphine who are physically opioid-dependent, initiate the taper by a small enough increment (e.g., no greater than 10% to 25% of the total daily dose) to avoid withdrawal symptoms, and proceed with dose-lowering at an interval of every 2 to 4 weeks. Patients who have been taking opioids for briefer periods of time may tolerate a more rapid taper.
It may be necessary to provide the patient with lower dosage strengths to accomplish a successful taper. Reassess the patient frequently to manage pain and withdrawal symptoms, should they emerge. Common withdrawal symptoms include restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. If withdrawal symptoms arise, it may be necessary to pause the taper for a period of time or raise the dose of the opioid analgesic to the previous dose, and then proceed with a slower taper. In addition, monitor patients for any changes in mood, emergence of suicidal thoughts, or use of other substances.
When managing patients taking opioid analgesics, particularly those who have been treated for a long duration and/or with high doses for chronic pain, ensure that a multimodal approach to pain management, including mental health support (if needed), is in place prior to initiating an opioid analgesic taper. A multimodal approach to pain management may optimize the treatment of chronic pain, as well as assist with the successful tapering of the opioid analgesic.
Dosage considerations
- Instruct patients to apply immediately after removal from the individually sealed pouch. Instruct patients not to use buprenorphine if the pouch seal is broken or the patch is cut, damaged, or changed in any way.
- Apply buprenorphine to the upper outer arm, upper chest, upper back or the side of the chest. These 4 sites (each present on both sides of the body) provide 8 possible application sites. Rotate buprenorphine among the 8 described skin sites. After buprenorphine removal, wait a minimum of 21 days before reapplying to the same skin site.
- Apply buprenorphine to a hairless or nearly hairless skin site. If none are available, the hair at the site should be clipped, not shaven. Do not apply BUTRANS to irritated skin. If the application site must be cleaned, clean the site with water only. Do not use soaps, alcohol, oils, lotions, or abrasive devices. Allow the skin to dry before applying buprenorphine.
- Incidental exposure of the buprenorphine patch to water, such as while bathing or showering is acceptable based on experience during clinical studies.
- If problems with adhesion of buprenorphine occur, the edges may be taped with first aid tape. If problems with lack of adhesion continue, the patch may be covered with waterproof or semipermeable adhesive dressings suitable for 7 days of wear.
- If buprenorphine falls off during the 7-day dosing interval, dispose of the transdermal system properly and place a new buprenorphine patch on at a different skin site.
- When changing the system, instruct patients to remove buprenorphine and dispose of it properly.
- If the buprenorphine-containing adhesive matrix accidentally contacts the skin, instruct patients or caregivers to wash the area with water and not to use soap, alcohol, or other solvents to remove the adhesive because they may enhance the absorption of the drug.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.