Adenocarcinoma of pancreas
Active Ingredient: Paclitaxel
Indication for Paclitaxel
Paclitaxel in combination with gemcitabine is indicated for the first-line treatment of adult patients with metastatic adenocarcinoma of the pancreas.
For this indication, competent medicine agencies globally authorize below treatments:
125 mg/m² on days 1, 8 and 15 of each 28-day cycle
Route of admnistration
Intravenous
Defined daily dose
125 - 125 mg per m² of body surface area (BSA)
Dosage regimen
From 125 To 125 mg per m² of body surface area (BSA) once every 7 day(s) for 15 day(s)
Detailed description
The recommended dose of paclitaxel in combination with gemcitabine is 125 mg/m² administered intravenously over 30 minutes on Days 1, 8 and 15 of each 28-day cycle. The concurrent recommended dose of gemcitabine is 1000 mg/m² administered intravenously over 30 minutes immediately after the completion of paclitaxel administration on Days 1, 8 and 15 of each 28-day cycle.
Dose adjustments during treatment of pancreatic adenocarcinoma
Dose level reductions for patients with pancreatic adenocarcinoma:
| Dose Level | Paclitaxel Dose (mg/m²) | Gemcitabine Dose (mg/m²) |
|---|---|---|
| Full dose | 125 | 1000 |
| 1st dose level reduction | 100 | 800 |
| 2nd dose level reduction | 75 | 600 |
| If additional dose reduction required | Discontinue treatment | Discontinue treatment |
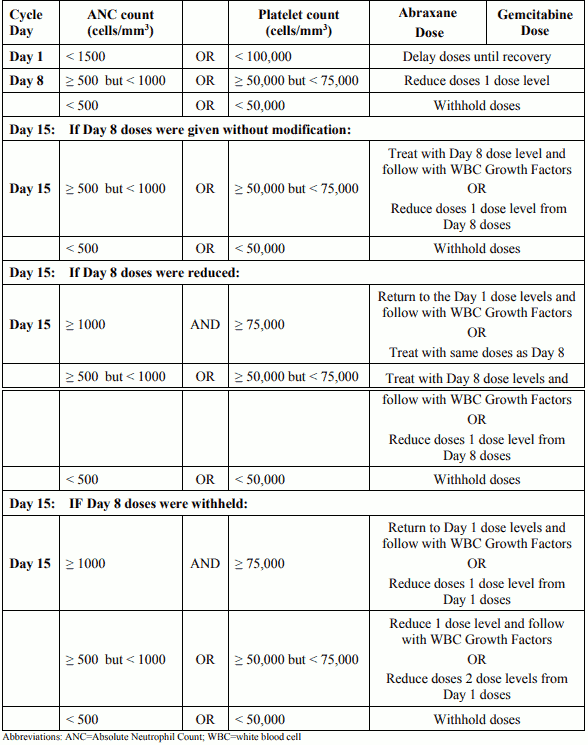
Dose modifications for neutropenia and/or thrombocytopenia at the start of a cycle or within a cycle for patients with pancreatic adenocarcinoma:
Dose modifications for other adverse drug reactions in patients with pancreatic adenocarcinoma:
| Adverse Drug Reaction (ADR) | Abraxane Dose | Gemcitabine Dose |
|---|---|---|
| Febrile Neutropenia: Grade 3 or 4 | Withhold doses until fever resolves and ANC ≥ 1500; resume at next lower dose levela | |
| Peripheral Neuropathy: Grade 3 or 4 | Withhold dose until improves to ≤ Grade 1; resume at next lower dose levela | Treat with same dose |
| Cutaneous Toxicity: Grade 2 or 3 | Reduce to next lower dose levela; discontinue treatment if ADR persists | |
| Gastrointestinal Toxicity: Grade 3 mucositis or diarrhoea | Withhold doses until improves to ≤ Grade 1; resume at next lower dose levela | |
a See table "Dose level reductions for patients with pancreatic adenocarcinoma" for dose level reductions
Dosage considerations
Administered intravenously over 30 minutes.
Liability Disclaimer : RxReasoner has utilized reasonable care in providing content and services that are accurate, complete and up to date. However, RxReasoner does not accept any responsibility or liability about it. The content and services of RxReasoner are for informational purposes only and they are not intended to be a substitute for the knowledge, expertise, skill, and judgment of physicians, pharmacists, nurses, or other healthcare professionals involved in patient care. RxReasoner offers no medical advice. Users are responsible for the use of the provided content. A shown indication or treatment should not be construed to indicate that the medication is safe, appropriate, or effective in any given patient or under any particular circumstances. The absence of an indication or treatment should not roule out the existence of other appropriate medications. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition or medicament. RxReasoner is not liable for any damages allegedly sustained arising out of the use of its content and services.