COPIKTRA Capsule Ref.[10038] Active ingredients: Duvelisib
Source: FDA, National Drug Code (US) Revision Year: 2019
12.1. Mechanism of Action
Duvelisib is an inhibitor of PI3K with inhibitory activity predominantly against PI3K-δ and PI3K-γ isoforms expressed in normal and malignant B-cells. Duvelisib induced growth inhibition and reduced viability in cell lines derived from malignant B-cells and in primary CLL tumor cells. Duvelisib inhibits several key cell-signaling pathways, including B-cell receptor signaling and CXCR12-mediated chemotaxis of malignant B-cells. Additionally, duvelisib inhibits CXCL12-induced T cell migration and M-CSF and IL-4 driven M2 polarization of macrophages.
12.2. Pharmacodynamics
At the recommended dose of 25 mg BID, reductions in levels of phosphorylated AKT (a downstream marker for PI3K inhibition) were observed in patients treated with COPIKTRA.
Cardiac Electrophysiology
The effect of multiple doses of COPIKTRA 25 and 75 mg BID on the QTc interval was evaluated in patients with previously treated hematologic malignancies. Increases of >20 ms in the QTc interval were not observed.
12.3. Pharmacokinetics
Duvelisib exposure increased in a dose-proportional manner over a dose range of 8 mg to 75 mg twice daily (0.3 to 3 times the recommended dosage).
At steady state following 25 mg BID administration of duvelisib in patients, the geometric mean (CV%) maximum concentration (Cmax) was 1.5 (64%) µg/mL and AUC was 7.9 (77%) µg•h/mL.
Absorption
The absolute bioavailability of 25 mg duvelisib after a single oral dose in healthy volunteers was 42%. The median time to peak concentration (Tmax) was observed at 1 to 2 hours in patients.
Effect of Food
COPIKTRA may be administered without regard to food. The administration of a single dose of COPIKTRA with a high-fat meal (fat accounted for approximately 50% of the total caloric content of the meal) decreased Cmax by approximately 37% and decreased the AUC by approximately 6%, relative to fasting conditions.
Distribution
Protein binding of duvelisib is greater than 98% with no concentration dependence. The mean blood-to-plasma ratio was 0.5. The geometric mean (CV%) apparent volume of distribution at steady state (Vss/F) is 28.5 L (62%). Duvelisib is a substrate of P-glycoprotein (P-gp) and BCRP in vitro.
Elimination
The geometric mean (CV%) apparent systemic clearance at steady-state is 4.2 L/hr (56%) in patients with lymphoma or leukemia. The geometric mean (CV%) terminal elimination half-life of duvelisib is 4.7 hours (57%).
Metabolism
Duvelisib is primarily metabolized by cytochrome P450 CYP3A4.
Excretion
Following a single 25 mg oral dose of radiolabeled duvelisib, 79% of the radioactivity was excreted in feces (11% unchanged) and 14% was excreted in the urine (<1% unchanged).
Specific Populations
Age (18 to 90 years), sex, race, renal impairment (creatinine clearance 23 to 80 mL/ min), hepatic impairment (Child Pugh Class A, B, and C) and body weight (40 to 154 kg) had no clinically significant effect on the exposure of duvelisib.
Drug Interaction Studies
Strong and Moderate CYP3A Inhibitors
Co-administration of strong CYP3A inhibitor ketoconazole (at 200 mg BID for 5 days), a strong inhibitor of CYP3A4, with a single oral 10 mg dose of COPIKTRA in healthy adults (n= 16) increased duvelisib Cmax by 1.7-fold and AUC by 4-fold. Based on physiologically-based pharmacokinetic (PBPK) modeling and simulation, the increase in exposure to duvelisib is estimated to be ~2-fold at steady state when concomitantly used with strong CYP3A4 inhibitors such as ketoconazole [see Dosage and Administration (2.3) and Drug Interactions (7.1)]. PBPK modeling and simulation estimated no effect on duvelisib exposures from concomitantly used mild or moderate CYP3A4 inhibitors.
Strong and Moderate CYP3A4 Inducers
Co-administration of 600 mg once daily rifampin, a strong CYP3A inducer, for 7 days with a single oral 25 mg COPIKTRA dose in healthy adults (N=13) decreased duvelisib Cmax by 66% and AUC by 82%.
The effect of moderate CYP3A4 induction has not been studied [see Drug Interactions (7.1)].
CYP3A4 Substrates
Co-administration of multiple doses of COPIKTRA 25 mg BID for 5 days with single oral 2 mg midazolam, a sensitive CYP3A4 substrate, in healthy adults (N=14), increased in the midazolam AUC by 4.3-fold and Cmax by 2.2-fold [see Drug Interactions (7.2)].
In Vitro Studies
Duvelisib is a substrate of P-glycoprotein (P-gp) and breast cancer-resistant protein (BCRP).
Duvelisib does not inhibit OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, BCRP, or P-gp.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with duvelisib.
Duvelisib did not cause genetic damage in in vitro or in vivo assays.
Fertility studies with duvelisib were not conducted. Histological findings in male and female rats were observed in the repeat dose toxicity studies and included testis (seminiferous epithelial atrophy, decreased weight, soft testes), and epididymis (small size, oligo/aspermia) in males and ovary (decreased weight) and uterus (atrophy) in females.
14. Clinical Studies
14.1 Efficacy in Relapsed or Refractory CLL/SLL
Study 1
A randomized, multicenter, open-label trial (Study 1; NCT02004522) compared COPIKTRA versus ofatumumab in 319 adult patients with CLL (N=312) or SLL (N=7) after at least one prior therapy. The trial excluded patients with prior autologous transplant within 6 months or allogeneic transplant, prior exposure to a PI3K inhibitor or a Bruton’s tyrosine kinase (BTK) inhibitor. The trial required hepatic transaminases ≤3 times upper limit of normal (ULN), total bilirubin ≤1.5 times ULN, and serum creatinine ≤2 times ULN.
The study randomized patients with a 1:1 ratio to receive either COPIKTRA 25 mg BID until disease progression or unacceptable toxicity or ofatumumab for 7 cycles. Ofatumumab was administered intravenously at an initial dose of 300 mg, followed one week later by 2000 mg once weekly for 7 doses, and then 2000 mg once every 4 weeks for 4 additional doses.
The approval of COPIKTRA was based on efficacy and safety analysis of patients with at least 2 prior lines of therapy, where the benefit:risk appeared greater in this more heavily pretreated population compared to the overall trial population.
In this subset (95 randomized to COPIKTRA, 101 to ofatumumab), the median patient age was 69 years (range: 40 to 90 years), 59% were male, and 88% had an ECOG performance status of 0 or 1. Forty-six percent received 2 prior lines of therapy, and 54% received 3 or more prior lines. At baseline, 52% of patients had at least one tumor ≥5 cm, and 22% of patients had a documented 17p deletion.
During randomized treatment, the median duration of exposure to COPIKTRA was 13 months (range: 0.2 to 37), with 80% of patients receiving at least 6 months and 52% receiving at least 12 months of COPIKTRA. The median duration of exposure to ofatumumab was 5 months (range: <0.1 to 6).
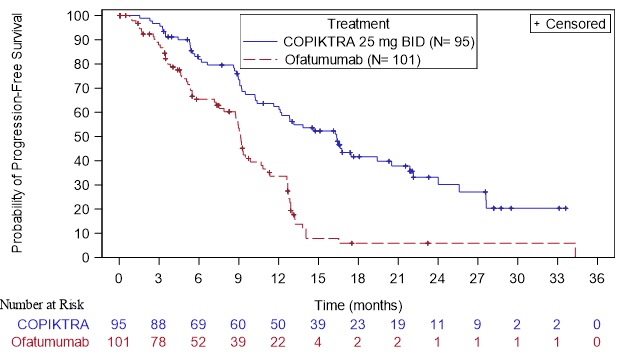
Efficacy was based on progression-free survival (PFS) as assessed by an Independent Review Committee (IRC). Other efficacy measures included overall response rate. Efficacy of COPIKTRA compared to ofatumumab specifically in patients treated with at least two prior therapies is presented in Table 8 and Figure 1.
Table 8. Efficacy in CLL or SLL After at Least Two Prior Therapies (Study 1):
| Outcome per IRC | COPIKTRA N=95 | Ofatumumab N=101 |
|---|---|---|
| PFS | ||
| Number of events, n (%) | 55 (58) | 70 (69) |
| Progressive disease | 44 | 62 |
| Death | 11 | 8 |
| Median PFS (SE), monthsa | 16.4 (2.1) | 9.1 (0.5) |
| Hazard Ratio (SE)b, COPIKTRA/ofatumumab | 0.40 (0.2) | |
| Response rate | ||
| ORR, n (%)c | 74 (78) | 39 (39) |
| CR | 0 (0) | 0 (0) |
| PR | 74 (78) | 39 (39) |
| Difference in ORR, % (SE) | 39 (6.4) | |
Abbreviations: CI = confidence interval; CR = complete response; IRC = Independent Review Committee; PFS = progression-free survival; PR = partial response; SE = standard error
a Kaplan-Meier estimate
b Standard Error of ln(hazard ratio) = 0.2
c IWCLL or revised IWG response criteria, with modification for treatment-related lymphocytosis
Figure 1. Kaplan-Meier Curve of PFS per IRC In Patients with at Least 2 Prior Therapies (Study 1):
14.2 Efficacy in Relapsed or Refractory FL
Study 2
Efficacy of COPIKTRA in patients with previously treated FL is based on a single-arm, multicenter trial (Study 2; NCT01882803). In this study, COPIKTRA 25 mg BID was administered in patients with FL (N=83) who were refractory to rituximab and to either chemotherapy or radioimmunotherapy. Refractory disease was defined as less than a partial remission or relapse within 6 months after the last dose. The trial excluded patients with Grade 3b FL, large cell transformation, prior allogeneic transplant, and prior exposure to a PI3K inhibitor or to a Bruton’s tyrosine kinase inhibitor.
The median age was 64 years (range: 30 to 82 years), 68% were male, and 37% had bulky disease assessed at baseline (target lesion ≥5 cm). Patients had a median of 3 prior lines of therapy (range: 1 to 10), with 94% being refractory to their last therapy and 81% being refractory to 2 or more prior lines of therapy. Most patients (93%) had an ECOG performance status of 0 or 1.
The median duration of exposure to COPIKTRA was 5 months (range: 0.4 to 24), with 41% of patients receiving at least 6 months and 10% receiving at least 12 months of COPIKTRA.
Efficacy was based on overall response rate and duration of response as assessed by an IRC (Table 9).
Table 9. Efficacy in Patients with Relapsed or Refractory FL (Study 2):
| Endpoint | FL N=83 |
|---|---|
| ORR, n (%)a | 35 (42) |
| 95% CI | (31, 54) |
| CR, n (%) | 1 (1) |
| PR, n (%) | 34 (41) |
| Duration of response | |
| Range, months | 0.0 to 41.9 |
| Patients maintaining response at 6 months, n/N (%) | 15/35 (43) |
| Patients maintaining response at 12 months, n/N (%) | 6/35 (17) |
Abbreviations: CI = confidence interval; CR = complete response; IRC = Independent Review Committee; ORR = overall response rate; PR = partial response
a Per IRC according to Revised International Working Group criteria
+ Denotes censored observation
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.