QINLOCK Tablet Ref.[10190] Active ingredients: Ripretinib
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Ripretinib is a tyrosine kinase inhibitor that inhibits KIT proto-oncogene receptor tyrosine kinase (KIT) and platelet derived growth factor receptor A (PDGFRA) kinase, including wild type, primary, and secondary mutations. Ripretinib also inhibits other kinases in vitro, such as PDGFRB, TIE2, VEGFR2, and BRAF.
12.2. Pharmacodynamics
Exposure-Response Relationships
Ripretinib exposure-response relationships and the time course of pharmacodynamics have not been fully characterized.
Cardiac Electrophysiology
No large mean increase in QTc interval (i.e. >20 ms) was detected following treatment with QINLOCK at the recommended dose of 150 mg taken orally once daily.
12.3. Pharmacokinetics
The pharmacokinetics of ripretinib and its equally active metabolite (DP-5439) were evaluated following single doses in healthy subjects and multiple doses in patients with advanced malignancies; the results are summarized in Table 5.
Table 5. Pharmacokinetic Parameters of Ripretinib and DP-5439:
| Parameter | Ripretinib | DP-5439 | |
|---|---|---|---|
| General Information | |||
| Steady state exposure following QINLOCK 150 mg once daily [Mean (CV%)] | Cmax (ng/mL) | 761 (32) | 804 (46) |
| AUC0-12h (ng•h/mL) | 5678 (32) | 7138 (44) | |
| Dose proportionality following single doses of QINLOCK in patients with advanced malignancies: | AUC0-24h increased proportionally over a dose range of 20-250 mg (0.13 to 1.67 times the recommended dose), but Cmax was less than dose proportional. | Cmax and AUC0-24h were less than dose proportional within the dose range of 50-250 mg (0.33 to 1.67 times the recommended dose). | |
| Time to steady state [Days] | 14 | 14 | |
| Accumulation ratio (AUC0-12h) [Mean (CV%)] a | 1.7 (55) | 5.29 (49) | |
| Absorption | |||
| Tmax [Median in hours] b | 4 | 15.6 | |
| Effect of Food | No clinically significant differences in the Cmax and AUC0-24h were observed between administration of QINLOCK with a high-fat meal c and under fasted conditions. | ||
| Distribution | |||
| Plasma protein binding (in vitro) | Human serum albumin | 99.8% | 99.7% |
| α-1 acid glycoprotein | 99.4% | >99.8% | |
| Steady state apparent volume of distribution, L [Mean (CV%)] b | 307 (39) | 507 (51) | |
| Elimination | |||
| Apparent clearance, L/hr [Mean (CV%)] b | 15.3 (45) | 17.5 (63) | |
| Half-life, hours [Mean (CV%)] b | 14.8 (30) | 17.8 (23) | |
| Metabolism | |||
| Metabolic pathways | Major | CYP3A4 | CYP3A4 |
| Minor | CYP2C8 and CYP2D6 | CYP2C8, CYP2E1 and CYP2D6 | |
| Excretion b | |||
| Excretion pathways | Feces | 34% | 6% |
| Urine | 0.02% | 0.1% | |
a Estimated based on cycle 1, day 15
b After a single oral dose of 150 mg
c A high fat meal consisted of approximately 150, 250, and 500-600 calories from protein, carbohydrate, and fat, respectively
CV=coefficient of variation; Cmax=maximum plasma concentration; AUC0-12h=area under the plasma concentration-time curve from time zero to 12 hours; AUC0-24h=area under the plasma concentration-time curve from time zero to 24 hours; Tmax=time to maximum concentration
Specific Populations
No clinically significant differences in the pharmacokinetics of ripretinib were observed based on age (19 to 87 years), sex, race (White, Black, and Asian), body weight (39 to 138 kg), tumor (GIST or other solid tumors), prior gastrectomy, mild to moderate renal impairment (CLcr 30 to <90 mL/min estimated by Cockcroft-Gault), and mild hepatic impairment (total bilirubin ≤ULN and AST >ULN or total bilirubin 1 to 1.5 × ULN and AST any). The effects of severe renal impairment (CLcr 15 to 29 mL/min) or moderate to severe hepatic impairment (total bilirubin >1.5 × ULN, AST any) on the pharmacokinetics of ripretinib have not been studied.
Drug Interaction Studies
Clinical Studies
Strong CYP3A Inhibitors: Coadministration of QINLOCK with itraconazole (a strong CYP3A inhibitor and also a P-gp inhibitor) increased ripretinib Cmax by 36% and AUC0-INF by 99% and also increased DP-5439 AUC0-INF by 99% with no change in its Cmax.
Strong CYP3A Inducers: The effect of coadministration of QINLOCK with a strong CYP3A inducer has not been studied. Ripretinib and DP-5439 are metabolized by CYP3A.
Proton Pump Inhibitors: No clinically significant differences in the plasma exposure to ripretinib and DP-5439 were observed when QINLOCK was coadministered with pantoprazole (a proton pump inhibitor).
In Vitro Studies
CYP Enzymes: Ripretinib and DP-5439 are inhibitors of CYP2C8. Ripretinib and DP-5439 are not inducers of CYP1A2, CYP2B6, or CYP3A4.
Transporter Systems: Ripretinib is an inhibitor of P-gp (P-glycoprotein) and BCRP (Breast Cancer Resistance Protein). DP-5439 is a substrate for P-gp and BCRP. DP-5439 is an inhibitor of BCRP and MATE1 (Multidrug And Toxin Extrusion Protein 1).
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with ripretinib.
Ripretinib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay or clastogenic in either an in vitro human lymphocyte culture micronucleus assay or an in vivo rat bone marrow micronucleus assay.
Dedicated fertility studies in male animals were not conducted with ripretinib. Findings in male reproductive organs occurred in repeat-dose toxicity studies and included degeneration of the testes and cellular debris of the epididymis in males administered ≥30 mg/kg/day (approximately one half of the human exposure at the recommended dose of 150 mg).
14. Clinical Studies
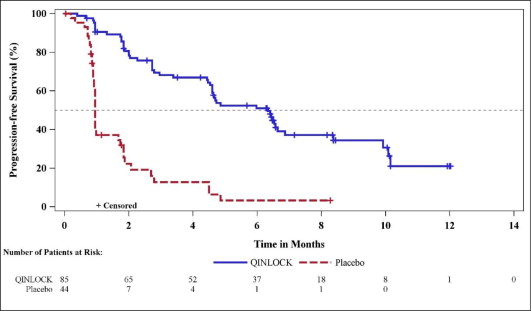
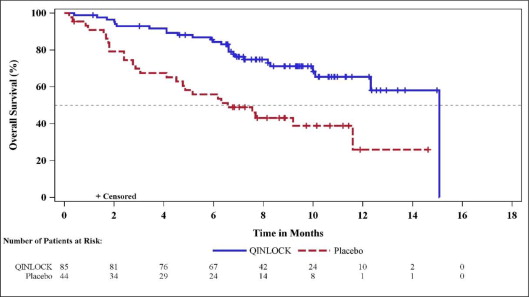
The efficacy of QINLOCK was evaluated in INVICTUS, an international, multi-center, randomized (2:1), double-blind, placebo-controlled trial (NCT03353753). Eligible patients had unresectable, locally advanced or metastatic gastrointestinal stromal tumor (GIST) and had received prior treatment with imatinib, sunitinib, and regorafenib. Randomization was stratified by prior lines of therapy (3 versus ≥4) and Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1 or 2). Patients received QINLOCK 150 mg or placebo orally once daily until disease progression or unacceptable toxicity. Tumor response assessments were performed every 28 days through for the first 4 months and then every 56 days thereafter. The major efficacy outcome measure was progression-free survival (PFS) based on disease assessment by blinded independent central review (BICR) using modified RECIST 1.1 criteria, in which lymph nodes and bone lesions were not target lesions and a progressively growing new tumor nodule within a pre-existing tumor mass must meet specific criteria to be considered unequivocal evidence of progression. Additional efficacy outcome measures included objective response rate (ORR) by BICR and overall survival (OS). Patients randomized to receive placebo could be treated with QINLOCK at the time of disease progression.
A total of 129 patients were randomized, 85 to QINLOCK and 44 to placebo.
Patient characteristics of the intent-to-treat (ITT) population in INVICTUS were median age of 60 years (range: 29 to 83 years), with 39% aged ≥65 years; 57% were male; 75% were White; and 92% had an ECOG performance status of 0 or 1. Sixty-three percent (63%) of patients received 3 prior therapies and 37% received 4 or more prior therapies. Sixty-six percent (66%) of patients randomized to placebo switched to QINLOCK after disease progression.
Efficacy results from INVICTUS are summarized in Table 6.
Table 6. Efficacy Results of INVICTUS:
| QINLOCK (N=85) | Placebo (N=44) | |
|---|---|---|
| Progression-Free Survivala | ||
| Number of events (%) | 51 (60) | 37 (84) |
| Progressive disease | 46 (54) | 32 (73) |
| Deaths | 5 (6) | 5 (11) |
| Median PFS (months) (95% CI) | 6.3 (4.6, 6.9) | 1.0 (0.9, 1.7) |
| Hazard ratio (95% CI)c | 0.15 (0.09, 0.25) | |
| p-valueb | < 0.0001 | |
| Overall Response Ratea | ||
| Overall Response Rate (%) | 9 | 0 |
| (95% CI) | (4.2, 18) | (0, 8) |
| p-value d | 0.0504 | |
| Overall Survivale | ||
| Number of deaths (%) | 26 (31) | 26 (59) |
| Median OS (months) (95% CI) | 15.1 (12.3, 15.1) | 6.6 (4.1, 11.6) |
| Hazard ratio (95% CI)c | 0.36 (0.21, 0.62) | |
BICR=Blinded Independent Central Review; CI=Confidence Interval
a Assessed per BICR.
b p-value is based on 2-sided stratified log-rank test.
c Hazard ratio is based on Cox proportional regression model. This model includes treatment and randomization stratification factors as fixed factors.
d Based on Fisher's exact test. The p-value is not statistically significant.
e Not evaluated for statistical significance as a result of the sequential testing procedure for the secondary endpoints of ORR and OS.
Figure 1. Kaplan-Meier Curve of Progression-Free Survival in INVICTUS:
Figure 2. Kaplan-Meier Curve of Overall Survival in INVICTUS:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.