EVRYSDI Oral solution Ref.[10192] Active ingredients: Risdiplam
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Risdiplam is a survival of motor neuron 2 (SMN2) splicing modifier designed to treat patients with spinal muscular atrophy (SMA) caused by mutations in chromosome 5q that lead to SMN protein deficiency. Using in vitro assays and studies in transgenic animal models of SMA, risdiplam was shown to increase exon 7 inclusion in SMN2 messenger ribonucleic acid (mRNA) transcripts and production of full-length SMN protein in the brain.
In vitro and in vivo data indicate that risdiplam may cause alternative splicing of additional genes, including FOXM1 and MADD. FOXM1 and MADD are thought to be involved in cell cycle regulation and apoptosis, respectively, and have been identified as possible contributors to adverse effects seen in animals.
12.2. Pharmacodynamics
In clinical trials, EVRYSDI led to an increase in SMN protein with a greater than 2-fold median change from baseline within 4 weeks of treatment initiation. The increase was sustained throughout the treatment period (of at least 12 months) across all SMA types.
12.3. Pharmacokinetics
Pharmacokinetics of EVRYSDI have been characterized in healthy adult subjects and in patients with SMA.
After administration of EVRYSDI as an oral solution, pharmacokinetics of risdiplam were approximately linear between 0.6 and 18 mg in a single-ascending-dose study in healthy adult subjects, and between 0.02 and 0.25 mg/kg once daily in a multiple-ascending-dose study in patients with SMA. Following once-daily oral administration of risdiplam in healthy subjects, approximately 3-fold accumulation of peak plasma concentrations (Cmax) and area under the plasma concentration-time curve (AUC0-24h) was observed. Risdiplam exposures reach steady state 7 to 14 days after once-daily administration.
Absorption
Following oral administration, the time to reach maximum plasma concentration (Tmax) is between 1 and 4 hours.
Effect of Food
In the clinical efficacy studies (Study 1 and Study 2), risdiplam was administered with a morning meal or after breastfeeding.
Distribution
The apparent volume of distribution at steady state is 6.3 L/kg.
Risdiplam is predominantly bound to serum albumin, without any binding to alpha-1 acid glycoprotein, with a free fraction of 11%.
Elimination
The apparent clearance (CL/F) of risdiplam is 2.1 L/h for a 14.9 kg patient.
The terminal elimination half-life of risdiplam was approximately 50 hours in healthy adults.
Metabolism
Risdiplam is primarily metabolized by flavin monooxygenase 1 and 3 (FMO1 and FMO3) and also by CYPs 1A1, 2J2, 3A4, and 3A7.
Parent drug was the major component found in plasma, accounting for 83% of drug-related material in circulation. The pharmacologically-inactive metabolite M1 was identified as the major circulating metabolite.
Excretion
Following a dose of 18 mg, approximately 53% of the dose (14% unchanged risdiplam) was excreted in the feces and 28% in urine (8% unchanged risdiplam).
Specific Populations
There were no clinically significant differences in the pharmacokinetics of EVRYSDI based on race or gender. Renal impairment is not expected to alter the exposures to risdiplam.
The impact of geriatric age and hepatic impairment on the pharmacokinetics of EVRYSDI has not been studied.
Pediatric Patients
Body weight and age were found to have significant effect on the pharmacokinetics of risdiplam. The estimated exposure (mean AUC0-24h) for infantile-onset SMA patients (age 2 to 7 months at enrollment) at the recommended dose of 0.2 mg/kg once daily was 1930 ng.h/mL. The estimated exposure for later-onset SMA patients (2 to 25 years old at enrollment) at the recommended dose was 2050 ng.h/mL (0.25 mg/kg once daily for patients with a body weight <20 kg and 5 mg once daily for patients with a body weight ≥20 kg). The observed maximum concentration (mean Cmax) was 184 ng/mL for infantile-onset SMA patients and 148 ng/mL for later-onset SMA patients.
Based on literature reports, pediatric patients less than 2 months of age are expected to have reduced activity of FMO3, which may result in increased exposure to risdiplam [see Elimination]. No data on risdiplam pharmacokinetics are available in patients less than 2 months of age [see Use in Specific Populations (8.4)].
Drug Interaction Studies
Effect of Other Drugs on EVRYSDI
Coadministration of 200 mg itraconazole (a strong CYP3A inhibitor) twice daily with a single 6 mg oral dose of risdiplam did not have a clinically relevant effect on the pharmacokinetics of risdiplam (11% increase in AUC and 9% decrease in Cmax).
Risdiplam is a weak substrate of human MDR-1 and breast cancer resistant protein (BCRP) transporters in vitro. Human MDR-1 or BCRP inhibitors are not expected to result in a clinically significant increase of risdiplam concentrations.
Effect of EVRYSDI on Other Drugs
Risdiplam and its major circulating metabolite M1 did not induce CYP1A2, 2B6, 2C8, 2C9, 2C19, or 3A4 in vitro. Risdiplam and M1 did not inhibit (reversible or time-dependent inhibition) any of the CYP enzymes tested (CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6), with the exception of CYP3A in vitro.
EVRYSDI is a weak inhibitor of CYP3A. In healthy adult subjects, administration of EVRYSDI once daily for 2 weeks slightly increased the exposure of midazolam, a sensitive CYP3A substrate (AUC 11%; Cmax 16%); this increase is not considered clinically relevant. Based on physiologically-based pharmacokinetic (PBPK) modeling, a similar increase is expected in children and infants as young as 2 months of age.
In vitro studies have shown that risdiplam and its major metabolite are not significant inhibitors of human MDR1, organic anion-transporting polypeptide (OATP) 1B1, OATP1B3, organic anion transporter 1 and 3 (OAT 1 and 3) transporters, and human organic cation transporter 2 (OCT2), at clinically relevant concentrations. Risdiplam and its metabolite are, however, in vitro inhibitors of the multidrug and toxin extrusion (MATE) 1 and MATE2-K transporters [see Drug Interactions (7.1)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of risdiplam has not been fully evaluated. Risdiplam was not carcinogenic in Tg.rasH2 mice when administered at oral doses of up to 9 mg/kg/day for 26 weeks.
Mutagenesis
Risdiplam was negative in an in vitro Ames assay. In an in vivo combined bone marrow micronucleus and comet assay in rat, risdiplam was clastogenic, as evidenced by an increase in micronuclei in bone marrow, but was negative in the comet assay. A pronounced increase in bone marrow micronuclei was also observed in toxicity studies in adult and juvenile rats [see Use in Specific Populations (8.4)].
Impairment of Fertility
Oral administration of risdiplam to rats for 4 (0, 1, 3, or 9 mg/kg/day) or 26 (0, 1, 3, or 7.5 mg/kg/day) weeks resulted in histopathological effects in the testis (degenerated spermatocytes, degeneration/atrophy of the seminiferous tubules) and epididymis (degeneration/necrosis of ductular epithelium) at the mid and/or high doses. At the high dose in the 26-week study, the testicular lesions persisted to the end of the recovery period, which corresponds, in rat, to approximately one spermatogenic cycle. The no-effect dose for adverse reproductive system effects in adult male rats (1 mg/kg/day) was associated with plasma drug exposures (AUC) similar to that in humans at the maximum recommended human dose (MRHD) of 5 mg/day.
Adverse effects of risdiplam on the testis could not be fully evaluated in the monkey because the majority of monkeys tested were sexually immature. However, oral administration of risdiplam (0, 2, 4, or 6 mg/kg/day) for 2 weeks resulted in histopathological changes in the testis (increases in multinucleate cells, germ cell degeneration) at the highest dose. At the no-effect dose for testicular toxicity in monkeys, plasma exposures were approximately 3 times that in humans at the MRHD.
Oral administration of risdiplam to postweaning juvenile rats resulted in male reproductive toxicity (degeneration/necrosis of the testis seminiferous epithelium with associated oligo/aspermia in the epididymis and abnormal sperm parameters). The no-effect dose for adverse reproductive effects in postweaning male juvenile rats was associated with plasma exposures approximately 4 times that in humans at the MRHD [see Use in Specific Populations (8.4)].
13.2. Animal Toxicology and/or Pharmacology
Retinal toxicity
Risdiplam-induced functional and structural retinal abnormalities were seen in animal studies. In a 39-week toxicity study in monkeys, oral administration of risdiplam (0, 1.5, 3, or 7.5/5 mg/kg/day; high dose lowered after 4 weeks) produced functional abnormalities on the electroretinogram (ERG) in all mid- and high-dose animals at the earliest examination time (Week 20). These findings were associated with retinal degeneration, detected by optical coherence tomography (OCT), on Week 22, the first examination time. The retinal degeneration, with peripheral photoreceptor loss, was irreversible. A no-effect dose for the retinal findings (1.5 mg/kg/day) was associated with plasma exposures (AUC) similar to that in humans at the maximum recommended human dose (MRHD) of 5 mg.
Effect on Epithelial Tissues
Oral administration of risdiplam to rats and monkeys resulted in histopathological changes in epithelium of the gastrointestinal (GI) tract (apoptosis/single cell necrosis), lamina propria (vacuolation), the exocrine pancreas (single cell necrosis), the skin, tongue, and larynx (parakeratosis/hyperplasia/degeneration) with associated inflammation. The skin and GI epithelial effects were reversible. The no-effect doses for effects on epithelial tissues in rats and monkeys were associated with plasma exposures (AUC) similar to that in humans at the MRHD.
14. Clinical Studies
The efficacy of EVRYSDI for the treatment of patients with infantile-onset and later-onset SMA was evaluated in two clinical studies, Study 1 (NCT02913482) and Study 2 (NCT02908685).
The overall findings of these studies support the effectiveness of EVRYSDI in SMA patients 2 months of age and older and appear to support the early initiation of treatment with EVRYSDI.
14.1 Infantile-Onset SMA
Study 1 was an open-label, 2-part study to investigate the efficacy, safety, pharmacokinetics, and pharmacodynamics of EVRYSDI in patients with Type 1 SMA (symptom onset between 28 days and 3 months of age). Part 1 of Study 1 (n=21) provides efficacy and safety data in patients with Type 1 SMA. Additional safety information is provided by Part 2 of Study 1 (n=41) in patients with Type 1 SMA [see Adverse Reactions (6.1)].
In Part 1 of Study 1, patients (n=21) were enrolled in one of two dosage cohorts. Patients in the higher-dosage cohort (n=17) had their dosage adjusted to the recommended dosage of 0.2 mg/kg/day before 12 months of treatment, while patients in the low-dosage cohort (n=4) did not.
Effectiveness was established based on the ability to sit without support for at least 5 seconds (as measured by Item 22 of the Bayley Scales of Infant and Toddler Development – Third Edition (BSID-III) gross motor scale) and on the basis of survival without permanent ventilation. Permanent ventilation was defined as requiring a tracheostomy or more than 21 consecutive days of either non-invasive ventilation (≥16 hours per day) or intubation, in the absence of an acute reversible event.
The median age of onset of clinical signs and symptoms of Type 1 SMA in patients enrolled in Part 1 of Study 1 was 2.0 months (range: 0.9 to 3.0); 71% of patients were female, 81% were Caucasian, and 19% were Asian. The median age at enrollment was 6.7 months (range: 3.3 to 6.9), and the median time between onset of symptoms and first dose was 4.0 months (range: 2.0 to 5.8). All patients had genetic confirmation of homozygous deletion or compound heterozygosity predictive of loss of function of the SMN1 gene, and two SMN2 gene copies.
In Study 1 Part 1, the median duration of EVRYSDI treatment was 14.8 months (range: 0.6 to 26.0), and 19 patients were treated for a minimum duration of 12 months.
Of the patients who were treated with the recommended dosage of EVRYSDI 0.2 mg/kg/day, 41% (7/17) were able to sit independently for ≥ 5 seconds (BSID-III, Item 22) after 12 months of treatment. These results indicate a clinically meaningful deviation from the natural history of untreated infantile-onset SMA. As described in the natural history of untreated infantile-onset SMA, patients would not be expected to attain the ability to sit independently, and no more than 25% of these patients would be expected to survive without permanent ventilation beyond 14 months of age. After 12 months of treatment with EVRYSDI, 90% (19/21) of patients were alive without permanent ventilation (and reached 15 months of age or older). After a minimum of 23 months of treatment with EVRYSDI, 81% (17/21) of all patients were alive without permanent ventilation (and reached an age of 28 months or older; median 32 months; range 28 to 45 months).
14.2 Later-Onset SMA
Study 2 was a 2-part, multicenter trial to investigate the efficacy, safety, pharmacokinetics, and pharmacodynamics of EVRYSDI in patients diagnosed with SMA Type 2 or Type 3. Part 1 of Study 2 was dose-finding and exploratory in 51 patients (14% ambulatory). Part 2 was randomized, double-blind, placebo-controlled, and is described below.
The primary endpoint in Study 2 Part 2 was the change from baseline to Month 12 in the Motor Function Measure 32 (MFM32) score. A key secondary endpoint was the proportion of patients with a 3-point or greater change from baseline to Month 12 in the MFM32 total score. The MFM32 measures motor function abilities that relate to daily functions. The total MFM32 score is expressed as a percentage (range: 0 to 100) of the maximum possible score, with higher scores indicating greater motor function. Another key secondary endpoint was the Revised Upper Limb Module (RULM). The RULM is a tool used to assess motor performance of the upper limb in SMA patients. It tests proximal and distal motor functions of the arm. The total score ranges from 0 (all the items cannot be performed) to 37 (all the activities are achieved fully without any compensatory maneuvers).
Study 2 Part 2 enrolled 180 non-ambulatory patients with Type 2 (71%) or Type 3 (29%) SMA. Patients were randomized 2:1 to receive EVRYSDI at the recommended dosage [see Dosage and Administration (2.2)] or placebo. Randomization was stratified by age group (2 to 5, 6 to 11, 12 to 17, or 18 to 25 years of age).
The median age of patients at the start of treatment was 9.0 years (range 2 to 25), and the median time between onset of initial SMA symptoms and first treatment was 102.6 months (range 1 to 275). Of the 180 patients included in the trial, 51% were female, 67% were Caucasian, and 19% were Asian. At baseline, 67% of patients had scoliosis (32% of them with severe scoliosis). Patients had a mean baseline MFM32 score of 46.1, and RULM score of 20.1. Overall baseline demographic characteristics were reasonably balanced between the treatment groups (EVRYSDI and placebo), with the exception of scoliosis (63% in the EVRYSDI arm vs. 73% in the placebo group).
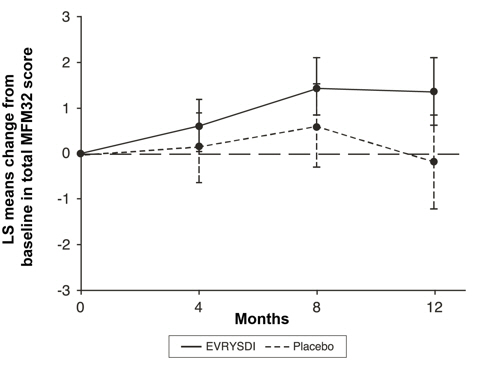
The primary analysis on the change from baseline in MFM32 total score at Month 12 showed a clinically meaningful and statistically significant difference between patients treated with EVRYSDI and placebo. The results of the primary analysis and key secondary endpoints are shown in Table 3 and Figure 1.
Table 3. Summary of Efficacy in Patients with Later-Onset SMA at Month 12 of Treatment (Study 2 Part 2):
| Endpoint | EVRYSDI (N=120) | Placebo (N=60) |
|---|---|---|
| Primary Endpoint: | ||
| Change from baseline in total MFM32 score at Month 12, LS means (95% CI)*,†,‡ | 1.36 (0.61, 2.11) | -0.19 (-1.22, 0.84) |
| Difference from Placebo, Estimate (95% CI)* p-value | 1.55 (0.30, 2.81) 0.0156 | |
| Secondary Endpoints: | ||
| Proportion of patients with a change from baseline MFM32 total score of 3 or more at Month 12 (95% CI)†,‡ | 38.3% (28.9, 47.6) | 23.7% (12.0, 35.4) |
| Odds ratio for overall response (95% CI) adjusted§ (unadjusted) p-value¶ | 2.35 (1.01, 5.44) 0.0469 (0.0469) | |
| Change from baseline in total score of RULM at Month 12, LS means (95% CI)*,# | 1.61 (1.00, 2.22) | 0.02 (-0.83, 0.87) |
| Difference from Placebo, Estimate (95% CI) adjusted§ (unadjusted) p-value* | 1.59 (0.55, 2.62) 0.0469 (0.0028) | |
* The Mixed Model Repeated Measure (MMRM) analysis included the change from baseline total score as the dependent variable and as independent variables the baseline total score, treatment group, time, treatment-by-time interaction, and the randomization stratification variable of age group (2 to 5, 6 to 11, 12 to 17, 18 to 25).
† The MFM total score was calculated according to the user manual, expressed as a percentage of the maximum score possible for the scale (i.e., sum of the 32 item scores divided by 96 and multiplied by 100).
‡ Based on the missing data rule for MFM32, 6 patients were excluded from the analysis (EVRYSDI n=115; placebo control n=59).
§ The adjusted p-value was obtained for the endpoints included in the hierarchical testing and was derived based on all the p-values from endpoints in order of the hierarchy up to the current endpoint.
¶ The logistic regression analysis included the baseline total score, treatment and age group as independent variables.
# Based on the missing data rule for RULM, 3 patients were excluded from the analysis (EVRYSDI n=119; placebo control n=58).
Figure 1. Mean Change from Baseline in Total MFM32 Score Over 12 Months (Study 2 Part 2)*,†:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.