AMOXAPINE Tablet Ref.[10288] Active ingredients: Amoxapine
Source: FDA, National Drug Code (US) Revision Year: 2015
Product description
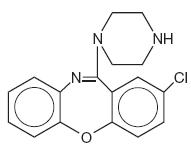
Amoxapine is an antidepressant of the dibenzoxazepine class, chemically distinct from the dibenzazepines, dibenzocycloheptenes, and dibenzoxepines.
It is designated chemically as 2-Chloro-11-(1-piperazinyl)dibenz[b,f][1,4]oxazepine. The structural formula is represented below:
C17H16CIN3O M.W. 313.78
Amoxapine is supplied for oral administration as 25 mg, 50 mg, 100 mg and 150 mg tablets.
Amoxapine Tablets USP, 25 mg, 50 mg, 100 mg and 150 mg contain: dibasic calcium phosphate, magnesium stearate, starch (corn), and stearic acid.
Amoxapine Tablets USP, 50 mg and 150 mg also contain: FD&C Yellow No. 6.
Amoxapine Tablets USP, 100 mg also contain: FD&C Blue No. 2.
| How Supplied |
|---|
|
Amoxapine Tablets USP, 25 mg are 8/32", scored, round, white tablets imprinted DAN 25 and 5713 supplied in bottles of 100. Amoxapine Tablets USP, 50 mg are 10/32", scored, round, orange tablets imprinted DAN 50 and 5714 supplied in bottles of 100. Amoxapine Tablets USP, 100 mg are 11/32", scored, round, blue tablets imprinted DAN 100 and 5715 supplied in bottles of 100. Amoxapine Tablets USP, 150 mg are 12/32", scored, round, orange tablets imprinted DAN 150 and 5716 supplied in bottles of 30. Dispense in a tight container with child-resistant closure. Manufactured by: Watson Pharma Private Limited, Verna, Salcette Goa 403 722 INDIA Distributed by: Actavis Pharma, Inc., Parsippany, NJ 07054 USA |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.