DAURISMO Film-coated tablet Ref.[10289] Active ingredients: Glasdegib
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Glasdegib is an inhibitor of the hedgehog pathway. Glasdegib binds to and inhibits Smoothened, a transmembrane protein involved in hedgehog signal transduction.
In a murine xenotransplant model of human AML, glasdegib in combination with low-dose cytarabine, inhibited increases in tumor size and reduced the percentage of CD45+/CD33+ blasts in the marrow to a greater extent than glasdegib or low-dose cytarabine alone.
12.2. Pharmacodynamics
Cardiac Electrophysiology
The effect of glasdegib administration on corrected QT interval (QTc) was evaluated in a randomized, single-dose, double-blind, 4-way crossover, placebo- and open-label moxifloxacin-controlled study in 36 healthy subjects. At therapeutic plasma concentrations for the recommended dose, achieved with a single dose of 150 mg DAURISMO, the largest placebo and baseline-adjusted QTc interval change was 8 ms (90% CI: 6, 10 ms). At a two-fold therapeutic plasma concentration, achieved with a single dose of 300 mg DAURISMO, the QTc change was 13 ms (90% CI: 11, 16 ms). Glasdegib is associated with concentration-dependent QTc prolongation.
12.3. Pharmacokinetics
DAURISMO at 5 mg to 600 mg once daily (0.05 to 6 times the recommended dose) result in a dose proportional increase in glasdegib peak concentrations (Cmax) and area under the curve over the dosing interval (AUC0-Tau). Steady-state plasma levels are reached by 8 days of daily dosing. The median accumulation ratio of glasdegib ranged from 1.2 to 2.5 following once-daily dosing.
At DAURISMO 100 mg once daily, the geometric mean (geometric coefficient of variation, % CV) of glasdegib Cmax was 1252 ng/mL (44%) and AUC0-Tau was 17210 ng*hr/mL (54%) in patients with cancer.
Absorption
The mean absolute bioavailability of DAURISMO is 77%. Following 100 mg once daily dosing, glasdegib median time to peak concentrations (Tmax) at steady-state ranged from 1.3 hours to 1.8 hours.
Effect of Food
A high-fat, high-calorie meal (total 800–1000 calories: 500–600 fat calories, 250 carbohydrate calories and 150 protein calories) reduced area under the curve over time to infinity (AUC0-INF) by 16% and Cmax by 31%.
Distribution
Glasdegib is 91% bound to human plasma proteins in vitro. The geometric mean (CV) apparent volume of distribution (Vz/F) was 188 L (20) in patients with hematologic malignancies.
Elimination
Glasdegib has a mean (± SD) half-life of 17.4 h (3.7) and geometric mean (CV) apparent clearance of 6.45 L/h (25) following 100 mg once daily dosing in patients with hematologic malignancies.
Metabolism
Glasdegib is primarily metabolized by the CYP3A4 pathway, with minor contributions by CYP2C8 and UGT1A9. Glasdegib accounts for 69% of the total circulating drug related material in plasma.
Excretion
Following a single oral dose of 100 mg radiolabeled glasdegib, 49% (17% unchanged) of the administered dose was eliminated in the urine and 42% (20% unchanged) of the administered dose was eliminated in the feces.
Specific Populations
Age (25 to 92 years), sex, race (White, Black, Asian), body weight (43.5 to 145.6 kg), mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin 1–1.5 × ULN and any AST), and mild renal impairment (creatinine clearance 60–89 mL/min) did not have clinically meaningful effects on the pharmacokinetics of glasdegib.
Patients with Renal Impairment
Following administration of a single dose of DAURISMO 100 mg, glasdegib AUC0-INF increased by 2.1-fold in subjects with moderate (eGFR 30 to 59 mL/min) and severe (eGFR 15 to 29 mL/min) renal impairment compared to subjects with normal renal function (eGFR ≥90 mL/min). The pharmacokinetics of glasdegib have not been studied in patients with end stage renal disease requiring hemodialysis.
Patients with Hepatic Impairment
Following administration of a single dose of DAURISMO 100 mg, glasdegib AUC0-INF increased by 11% in subjects with moderate hepatic impairment (Child-Pugh B) and decreased by 24% in subjects with severe hepatic impairment (Child-Pugh C) compared to subjects with normal hepatic function.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of Strong CYP3A4 Inhibitors on Glasdegib: Co-administration of ketoconazole (a strong inhibitor of CYP3A4) with DAURISMO increased the glasdegib AUC0-INF by 2.4-fold and Cmax by 1.4-fold over glasdegib given alone [see Drug Interactions (7)].
Effect of Strong and Moderate CYP3A4 Inducers on Glasdegib: Co-administration of rifampin (a strong inducer of CYP3A4) with DAURISMO decreased glasdegib AUC0-INF by 70% and Cmax by 35% [see Drug Interactions (7)]. Co-administration of efavirenz (moderate CYP3A4 inducer) is predicted to decrease glasdegib AUC0-INF by 55% and Cmax by 25%.
Effect of Gastric Acid Reducing Agents on Glasdegib: Co-administration of rabeprazole (a proton pump inhibitor) with DAURISMO did not alter glasdegib AUC0-INF but decreased Cmax by 20%.
In Vitro Studies
Effect of Glasdegib on Cytochrome P450 (CYP) Substrates: Glasdegib does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A, and does not induce CYP1A2, CYP2B6, and CYP3A in vitro.
Effect of Transporters on Glasdegib: Glasdegib is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP).
Effect of Glasdegib on Transporters: Glasdegib inhibits P-gp, BCRP, multidrug and toxin extrusion (MATE) protein 1, and MATE-2K, but not organic anion transporting polypeptide (OATP)1B1, OATP1B3, organic anion transporter (OAT)1, OAT3, and organic cation transporter (OCT)2 in vitro.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with glasdegib.
Glasdegib was not mutagenic in vitro in the bacterial reverse mutation (Ames) assay and was not clastogenic in the in vitro chromosome aberration assay in human lymphocytes. Glasdegib was not clastogenic or aneugenic in the rat micronucleus assay.
Based on nonclinical safety findings, glasdegib has the potential to impair reproductive function in males. Men should seek advice on effective fertility preservation before treatment. In repeat-dose toxicity studies in rats, findings observed in the male reproductive tract included adverse testicular changes with glasdegib at doses ≥50 mg/kg/day, and consisted of minimal to severe hypospermatogenesis characterized by partial to complete loss of spermatogonia, spermatocytes and spermatids and testicular degeneration. Hypospermatogenesis did not recover whereas testicular degeneration did recover. The dose at which testicular effects were observed in male rats was identified as 50 mg/kg/day with corresponding systemic exposures that were approximately 6.6-times (based on AUC) those associated with the observed human exposure at the 100 mg once daily dose.
14. Clinical Studies
The efficacy of DAURISMO in combination with low-dose cytarabine was evaluated in a multicenter, open-label, randomized study (Study BRIGHT AML 1003, NCT01546038) that included 115 patients age 55 years or older with newly-diagnosed AML who met at least one of the following criteria: a) age ≥75 years, b) severe cardiac disease, c) baseline Eastern Cooperative Oncology Group (ECOG) performance status of 2, or d) baseline serum creatinine >1.3 mg/dL. Patients were randomized 2:1 to receive DAURISMO at a 100 mg daily dose with low-dose cytarabine 20 mg subcutaneously twice daily on days 1 to 10 of a 28-day cycle (N=77) or low-dose cytarabine alone (N=38) in 28-day cycles until disease progression or unacceptable toxicity. Patients were stratified by cytogenetic risk (good/intermediate or poor).
The baseline demographic and disease characteristics are shown in Table 6. The two treatment arms were generally balanced with respect to the baseline demographics and disease characteristics (see Table 6).
Table 6. Baseline Demographic and Disease Characteristics in Patients with AML:
| Demographic and Disease Characteristics | DAURISMO With Low-Dose Cytarabine (N=77) | Low-Dose Cytarabine Alone (N=38) |
|---|---|---|
| Demographics | ||
| Age | ||
| Median (Min, Max) (Years) | 77 (64, 92) | 76 (58, 83) |
| ≥ 75 years N (%) | 47 (61) | 23 (61) |
| Sex, N (%) | ||
| Male | 59 (77) | 23 (61) |
| Female | 18 (23) | 15 (39) |
| Race, N (%) | ||
| White | 75 (97) | 38 (100) |
| Black or African American | 1 (1) | 0 (0) |
| Asian | 1 (1) | 0 (0) |
| Disease History, N (%) | ||
| De Novo AML | 38 (49) | 18 (47) |
| Secondary AML | 39 (51) | 20 (53) |
| Prior Hypomethylating Agent Use | 11 (14) | 6 (16) |
| ECOG PS?footnote?, N (%) | ||
| 0 to 1 | 35 (46) | 20 (53) |
| 2 | 41 (53) | 18 (47) |
| Cytogenetic Risk Status, N (%) | ||
| Good/Intermediate | 48 (62) | 21 (55) |
| Poor | 29 (38) | 17 (45) |
| Baseline Severe Cardiac Disease | 51 (66) | 20 (53) |
| Baseline Serum Creatinine >1.3 mg/dL | 15 (19) | 5 (13) |
Abbreviations: AML=acute myeloid leukemia; N=number of patients; ECOG PS=Eastern Cooperative Oncology Group Performance Status.
* Baseline ECOG PS was not reported for one patient in the DAURISMO with lowdose cytarabine arm.
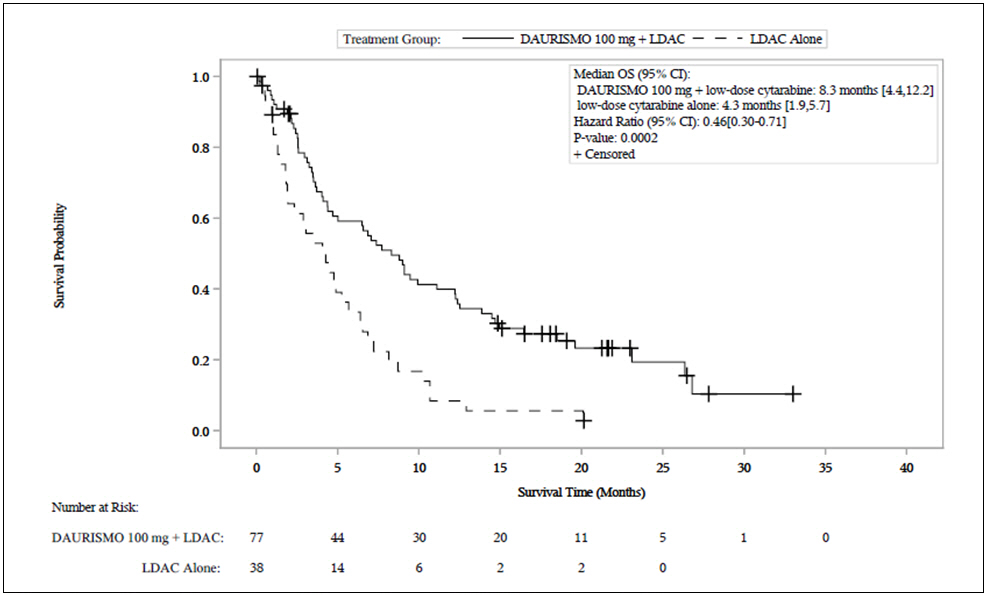
Efficacy was established on the basis of overall survival (OS) from the date of randomization to death from any cause. With a median follow-up of approximately 20 months, the DAURISMO with low-dose cytarabine arm was superior to low-dose cytarabine alone arm (Figure 1). The efficacy results are shown in Table 7. Improvement in OS was consistent across prespecified cytogenetic risk subgroups.
Table 7. Efficacy Results From BRIGHT AML 1003:
| Endpoint/Study Population | DAURISMO With Low-Dose Cytarabine | Low-Dose Cytarabine Alone |
|---|---|---|
| OS | N=77 | N=38 |
| Median survival, months (95% CI) | 8.3 (4.4, 12.2) | 4.3 (1.9, 5.7) |
| Hazard ratio (95% CI)* | 0.46 (0.30, 0.71) | |
| p-value† | 0.0002 | |
| CR | N=14 | N=1 |
| CR rate (in , 95 CI) | 18.2 (10.3, 28.6) | 2.6 (0.1, 13.8) |
Abbreviations: AML = acute myeloid leukemia; N = number of patients; OS = overall survival; CI = confidence interval; CR = complete response.
* Hazard ratio (DAURISMO with low-dose cytarabine/low-dose cytarabine alone) based on the Cox Proportional hazards model stratified by cytogenetic risk.
† 1-sided p-value from log-rank test stratified by cytogenetic risk.
Figure 1. BRIGHT AML 1003 – Kaplan-Meier Plot of Overall Survival for Patients with AML:
Abbreviations: CI = confidence interval; OS = overall survival; LDAC = low-dose cytarabine.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.