Source: FDA, National Drug Code (US) Revision Year: 2020
Pfizer-BioNTech COVID-19 Vaccine is authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older.
For intramuscular injection only.
Prior to Dilution:
Dilution:
THAWING PRIOR TO DILUTION:
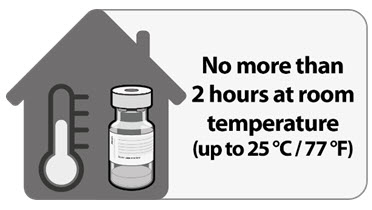 | • Thaw vial(s) of Pfizer-BioNTech COVID-19 Vaccine before use either by:•• Allowing vial(s) to thaw in the refrigerator [2ºC to 8ºC (35ºF to 46ºF)]. A carton of vials may take up to 3 hours to thaw, and thawed vials can be stored in the refrigerator for up to five days (120 hours). •• Allowing vial(s) to sit at room temperature [up to 25ºC (77ºF)] for 30 minutes. • Using either thawing method, vials must reach room temperature before dilution and must be diluted within 2 hours. |
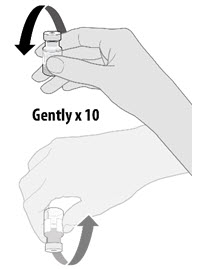 | • Before dilution invert vaccine vial gently 10 times. • Do not shake. • Inspect the liquid in the vial prior to dilution. The liquid is a white to off-white suspension and may contain white to off-white opaque amorphous particles. • Do not use if liquid is discolored or if other particles are observed. |
DILUTION:
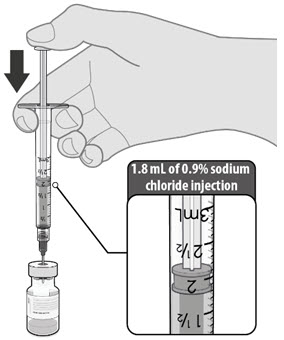 | • Obtain sterile 0.9% Sodium Chloride Injection, USP. Use only this as the diluent. • Using aseptic technique, withdraw 1.8 mL of diluent into a transfer syringe (21-gauge or narrower needle). • Cleanse the vaccine vial stopper with a single-use antiseptic swab. • Add 1.8 mL of 0.9% Sodium Chloride Injection, USP into the vaccine vial. |
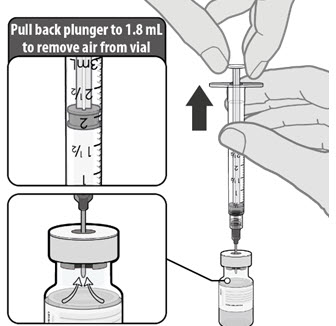 | • Equalize vial pressure before removing the needle from the vial by withdrawing 1.8 mL air into the empty diluent syringe. |
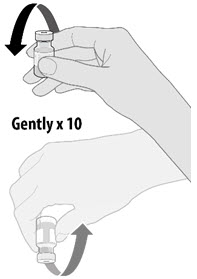 | • Gently invert the vial containing the Pfizer-BioNTech COVID-19 Vaccine 10 times to mix. • Do not shake. • Inspect the vaccine in the vial. • The vaccine will be an off-white suspension. Do not use if vaccine is discolored or contains particulate matter. |
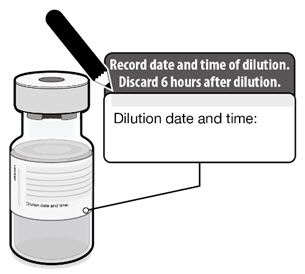 | • Record the date and time of dilution on the Pfizer-BioNTech COVID-19 Vaccine vial label. • Store between 2°C to 25°C (35°F to 77°F). • Discard any unused vaccine 6 hours after dilution. |
PREPARATION OF INDIVIDUAL 0.3 mL DOSES OF PFIZER-BIONTECH COVID-19 VACCINE:
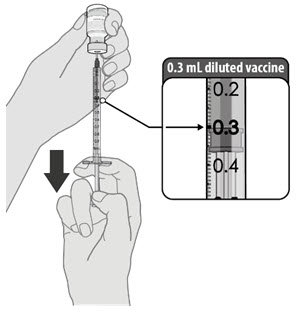 | • Using aseptic technique, cleanse the vial stopper with a single-use antiseptic swab, and withdraw 0.3 mL of the Pfizer-BioNTech COVID-19 Vaccine. • Administer immediately. |
Visually inspect each dose in the dosing syringe prior to administration. The vaccine will be an off-white suspension. During the visual inspection,
Administer the Pfizer-BioNTech COVID-19 Vaccine intramuscularly.
The Pfizer-BioNTech COVID-19 Vaccine is administered intramuscularly as a series of two doses (0.3 mL each) three weeks apart.
There are no data available on the interchangeability of the Pfizer-BioNTech COVID-19 Vaccine with other COVID-19 vaccines to complete the vaccination series. Individuals who have received one dose of Pfizer-BioNTech COVID-19 Vaccine should receive a second dose of Pfizer-BioNTech COVID-19 Vaccine to complete the vaccination series.
During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Do not refreeze thawed vials.
Cartons of Pfizer-BioNTech COVID-19 Vaccine Multiple Dose Vials arrive in thermal containers with dry ice. Once received, remove the vial cartons immediately from the thermal container and store in an ultra-low temperature freezer between -80ºC to -60ºC (-112ºF to -76ºF). Vials must be kept frozen between -80ºC to -60ºC (-112ºF to -76ºF) and protected from light, in the original cartons, until ready to use.
If an ultra-low temperature freezer is not available, the thermal container in which the Pfizer-BioNTech COVID-19 Vaccine arrives may be used as temporary storage when consistently re-filled to the top of the container with dry ice. Refer to the re-icing guidelines packed in the original thermal container for instructions regarding the use of the thermal container for temporary storage. The thermal container maintains a temperature range of -90ºC to -60ºC (-130ºF to -76ºF). Storage within this temperature range is not considered an excursion from the recommended storage condition.
Thawed Under Refrigeration:
Thaw and then store undiluted vials in the refrigerator [2ºC to 8ºC (35ºF to 46ºF)] for up to 5 days (120 hours). A carton of 25 vials or 195 vials may take up to 2 or 3 hours, respectively, to thaw in the refrigerator, whereas a fewer number of vials will thaw in less time.
Thawed at Room Temperature:
For immediate use, thaw undiluted vials at room temperature [up to 25ºC (77ºF)] for 30 minutes. Thawed vials can be handled in room light conditions.
Vials must reach room temperature before dilution.
Undiluted vials may be stored at room temperature for no more than 2 hours.
After dilution, store vials between 2°C to 25°C (35°F to 77°F) and use within 6 hours from the time of dilution. During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light. Any vaccine remaining in vials must be discarded after 6 hours. Do not refreeze.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.