IMIPENEM AND CILASTATIN Powder for solution for injection Ref.[10466] Active ingredients: Imipenem and Cilastatin
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
Imipenem and Cilastatin for Injection, USP (I.V.) (imipenem and cilastatin) for Injection is a sterile formulation of imipenem, a penem antibacterial, and cilastatin, a renal dehydropeptidase inhibitor with sodium bicarbonate added as a buffer. Imipenem and Cilastatin for Injection, USP (I.V.) is an antibacterial drug for intravenous administration.
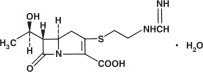
Imipenem (N-formimidoylthienamycin monohydrate) is a crystalline derivative of thienamycin, which is produced by Streptomyces cattleya. Its chemical name is (5R,6S)3[[2-(formimidoylamino)ethyl]thio]6[(R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid monohydrate. It is an off-white, nonhygroscopic crystalline compound with a molecular weight of 317.37. It is sparingly soluble in water and slightly soluble in methanol. Its empirical formula is C12H17N3O4S•H2O, and its structural formula is:
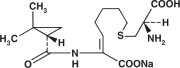
Cilastatin sodium is the sodium salt of a derivatized heptenoic acid. Its chemical name is sodium (Z)-7[[(R)-2-amino-2-carboxyethyl]thio]2[(S)-2,2-dimethylcyclopropanecarboxamido]-2-heptenoate. It is an off-white to yellowish-white, hygroscopic, amorphous compound with a molecular weight of 380.43. It is very soluble in water and in methanol. Its empirical formula is C16H25N2O5SNa, and its structural formula is:
Imipenem and Cilastatin for Injection, USP (I.V.) is buffered to provide solutions in the pH range of 6.5 to 8.5. There is no significant change in pH when solutions are prepared and used as directed. [see How Supplied/ Storage and Handling (16.1)]. Each Imipenem and Cilastatin for Injection, USP (I.V.) 250 mg/250 mg vial contains imipenem USP 250 mg (anhydrous equivalent) and cilastatin sodium USP equivalent to 250 mg cilastatin and each 500 mg/500 mg vial contains imipenem USP 500 mg (anhydrous equivalent) and cilastatin sodium USP equivalent to 500 mg cilastatin. In addition, the 250 mg/250 mg vial contains 10 mg of sodium bicarbonate and the 500 mg/500 mg vial contains 20 mg of sodium bicarbonate. The sodium content of the 250 mg/250 mg vial is 18.8 mg (0.8 mEq) and the sodium content for the 500 mg/500 mg vial is 37.5 mg (1.6 mEq). Solutions of Imipenem and Cilastatin for Injection, USP (I.V.) range from colorless to yellow. Variations of color within this range do not affect the potency of the product.
| Dosage Forms and Strengths |
|---|
|
For Injection Imipenem and Cilastatin for Injection (I.V.) is a sterile powder mixture for reconstitution in single-dose vials containing:
|
| How Supplied | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Imipenem and Cilastatin for Injection, USP (I.V.) is supplied as a sterile powder mixture in single-dose vials containing imipenem (anhydrous equivalent) and cilastatin (free acid equivalent) as follows:
Manufactured for: Fresenius Kabi USA, LLC, Lake Zurich, IL 60047 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.