ACIPHEX SPRINKLE Delayed-release capsule Ref.[10531] Active ingredients: Rabeprazole
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
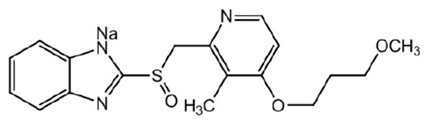
The active ingredient in ACIPHEX Sprinkle delayed-release capsules is rabeprazole sodium, which is a proton pump inhibitor. It is a substituted benzimidazole known chemically as 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1 H–benzimidazole sodium salt. It has an empirical formula of C18H20N3NaO3S and a molecular weight of 381.42. Rabeprazole sodium is a white to slightly yellowish-white solid. It is very soluble in water and methanol, freely soluble in ethanol, chloroform, and ethyl acetate and insoluble in ether and n-hexane. The stability of rabeprazole sodium is a function of pH; it is rapidly degraded in acid media, and is more stable under alkaline conditions. The structural figure is:
Figure 1:
ACIPHEX Sprinkle is available for oral administration as 5 mg and 10 mg rabeprazole sodium delayed-release capsules containing enteric coated granules.
ACIPHEX Sprinkle contains granules of rabeprazole sodium in a hard hypromellose capsule. Inactive ingredients are colloidal silicon dioxide, diacetylated monoglycerides, ethylcellulose, hydroxypropyl cellulose, hypromellose phthalate, magnesium oxide, magnesium stearate, mannitol, talc, titanium dioxide, carrageenan, potassium chloride, FD&C Blue No. 2 Aluminum Lake (in the 5 mg capsule), FD&C Yellow, No. 6 (in the 10 mg capsule), and gray printing ink.
| Dosage Forms and Strengths |
|---|
|
ACIPHEX Sprinkle is provided as:
|
| How Supplied |
|---|
|
ACIPHEX Sprinkle delayed-release capsules (5 mg) are supplied as transparent blue and opaque white capsules containing enteric coated granules. Identification and strength (ACX 5mg) are imprinted on the body of the capsule. An arrow (↑) imprint on the capsule cap indicates direction for opening a capsule. Bottles of 30 (NDC 23594-205-01) ACIPHEX Sprinkle delayed-release capsules (10 mg) are supplied as transparent yellow and opaque white capsules containing enteric coated granules. Identification and strength (ACX 10mg) are imprinted on the body of the capsule. An arrow (↑) imprint on the capsule cap indicates direction for opening a capsule. Bottles of 30 (NDC 23594-210-01) Distributed and Marketing by Cerecor, Inc. Rockville, MD 20850 ACIPHEX is a registered trademark of Eisai R&D Management Co., Ltd; |
Drugs
| Drug | Countries | |
|---|---|---|
| ACIPHEX | United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.