DIFLUCAN Tablet / Oral suspension Ref.[10562] Active ingredients: Fluconazole
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
DIFLUCAN (fluconazole), the first of a new subclass of synthetic triazole antifungal agents, is available as tablets for oral administration, as a powder for oral suspension.
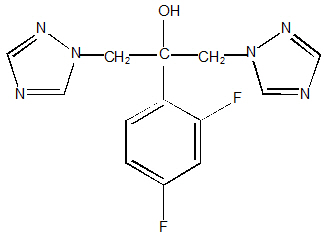
Fluconazole is designated chemically as 2,4-difluoro-α,α 1-bis(1H-1,2,4-triazol-1-ylmethyl) benzyl alcohol with an empirical formula of C13H12F2N6O and molecular weight of 306.3.
The structural formula is:
Fluconazole is a white crystalline solid which is slightly soluble in water and saline.
DIFLUCAN Tablets contain 50 mg, 100 mg, 150 mg, or 200 mg of fluconazole and the following inactive ingredients: microcrystalline cellulose, dibasic calcium phosphate anhydrous, povidone, croscarmellose sodium, FD&C Red No. 40 aluminum lake dye, and magnesium stearate.
DIFLUCAN for Oral Suspension contains 350 mg or 1400 mg of fluconazole and the following inactive ingredients: sucrose, sodium citrate dihydrate, citric acid anhydrous, sodium benzoate, titanium dioxide, colloidal silicon dioxide, xanthan gum, and natural orange flavor. After reconstitution with 24 mL of distilled water or Purified Water (USP), each mL of reconstituted suspension contains 10 mg or 40 mg of fluconazole.
| How Supplied |
|---|
|
DIFLUCAN Tablets: Pink trapezoidal tablets containing 50, 100, or 200 mg of fluconazole are packaged in bottles or unit dose blisters. The 150 mg fluconazole tablets are pink and oval shaped, packaged in a single dose unit blister. DIFLUCAN Tablets are supplied as follows: DIFLUCAN 50 mg Tablets: Engraved with "DIFLUCAN" and "50" on the front and "ROERIG" on the back. NDC 0049-3410-30 - Bottles of 30 DIFLUCAN 100 mg Tablets: Engraved with "DIFLUCAN" and "100" on the front and "ROERIG" on the back. NDC 0049-3420-30 - Bottles of 30 DIFLUCAN 150 mg Tablets: Engraved with "DIFLUCAN" and "150" on the front and "ROERIG" on the back. NDC 0049-3500-79 - Unit dose package of 1 DIFLUCAN 200 mg Tablets: Engraved with "DIFLUCAN" and "200" on the front and "ROERIG" on the back. NDC 0049-3430-30 - Bottles of 30 |
Drugs
| Drug | Countries | |
|---|---|---|
| DIFLUCAN | Austria, Australia, Canada, Germany, Ecuador, Estonia, Spain, Finland, Hong Kong, Croatia, Ireland, Israel, Italy, Japan, Lithuania, Malta, Mexico, Nigeria, Netherlands, New Zealand, Poland, Romania, Singapore, Tunisia, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.