PEPCID Oral suspension Ref.[10596] Active ingredients: Famotidine
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
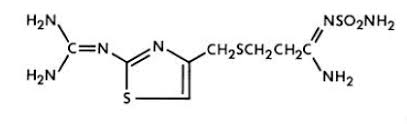
The active ingredient in PEPCID (famotidine) for oral suspension is a histamine-2 (H2) receptor antagonist. Famotidine is N'-3[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propanimidamide. The empirical formula of famotidine is C8H15N7O2S3 and its molecular weight is 337.43.
Its structural formula is:
Each 5 mL of PEPCID for oral suspension when prepared as directed contains 40 mg of famotidine and the following inactive ingredients: citric acid, flavors (cherry, banana, and mint), microcrystalline cellulose and carboxymethylcellulose sodium sucrose and xanthan gum. Added as preservatives are sodium benzoate 0.1%, sodium methylparaben 0.1% and sodium propylparaben 0.02%.
Famotidine is a white to pale yellow crystalline compound that is freely soluble in glacial acetic acid, slightly soluble in methanol, very slightly soluble in water, and practically insoluble in ethanol.
| Dosage Forms and Strengths |
|---|
|
For Oral Suspension: 400 mg as a white to off-white powder. When constituted as directed, PEPCID suspension is a smooth, mobile, off-white, homogeneous suspension with a cherry-banana-mint flavor, containing 40 mg of famotidine per 5 mL. |
| How Supplied | ||||||||
|---|---|---|---|---|---|---|---|---|
|
PEPCID (famotidine) for oral suspension is supplied as follows:
Prior to dispensing, constitute PEPCID for oral suspension [see Dosage and Administration (2.3)] Manufactured for: Bausch Health US, LLC, Bridgewater, NJ 08807 USA Manufactured by: Perrigo, Minneapolis, MN 55427 |
Drugs
| Drug | Countries | |
|---|---|---|
| PEPCID | Canada, Spain, Finland, Ireland, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.