PROZAC Capsule Ref.[10615] Active ingredients: Fluoxetine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
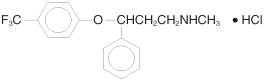
PROZAC (fluoxetine capsules, USP) is a selective serotonin reuptake inhibitor for oral administration. It is also marketed for the treatment of premenstrual dysphoric disorder (Sarafem, fluoxetine hydrochloride). It is designated (±)-N-methyl-3-phenyl-3-[(α,α,α-trifluoro-p-tolyl)oxy]propylamine hydrochloride and has the empirical formula of C17H18F3NO•HCl. Its molecular weight is 345.79.
The structural formula is:
Fluoxetine hydrochloride is a white to off-white crystalline solid with a solubility of 14 mg/mL in water.
Each Pulvule contains fluoxetine hydrochloride equivalent to 10 mg (32.3 μmol), 20 mg (64.7 μmol), or 40 mg (129.3 μmol) of fluoxetine. The Pulvules also contain starch, gelatin, silicone, titanium dioxide, iron oxide, and other inactive ingredients. The 10 and 20 mg Pulvules also contain FD&C Blue No. 1, and the 40 mg Pulvule also contains FD&C Blue No. 1 and FD&C Yellow No. 6.
| Dosage Forms and Strengths |
|---|
|
| How Supplied | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The following products are manufactured by Eli Lilly and Company for Dista Products Company: Pulvule are available in 10 mg, 20 mg and 40 mg capsule strengths and packages as follows:
1 Fluoxetine base equivalent. Marketed by: Lilly USA, LLC Indianapolis, IN 46285, USA |
|||||||||||||||||||||||||||||||||||||||||||
Drugs
| Drug | Countries | |
|---|---|---|
| PROZAC | Australia, Brazil, Canada, Ecuador, Estonia, Spain, France, Hong Kong, Ireland, Israel, Italy, Malta, Mexico, Netherlands, New Zealand, Romania, Singapore, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.