XALATAN Ophthalmic solution Ref.[10623] Active ingredients: Latanoprost
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
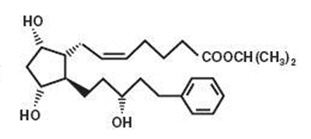
Latanoprost is a prostaglandin F2α analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is C26H40O5 and its chemical structure is:
M.W. 432.58
Latanoprost is a colorless to slightly yellow oil that is very soluble in acetonitrile and freely soluble in acetone, ethanol, ethyl acetate, isopropanol, methanol, and octanol. It is practically insoluble in water.
XALATAN (latanoprost ophthalmic solution) 0.005% is supplied as a sterile, isotonic, buffered aqueous solution of latanoprost with a pH of approximately 6.7 and an osmolality of approximately 267 mOsmol/kg. Each mL of XALATAN contains 50 mcg of latanoprost. Benzalkonium chloride, 0.02% is added as a preservative. The inactive ingredients are: sodium chloride, sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate anhydrous, and water for injection. One drop contains approximately 1.5 mcg of latanoprost.
| Dosage Forms and Strengths |
|---|
|
Ophthalmic solution containing latanoprost 50 mcg/mL (0.005%). |
| How Supplied |
|---|
|
XALATAN is a clear, isotonic, buffered, preserved colorless solution of latanoprost 50 mcg/mL (0.005%). It is supplied as a 2.5 mL solution in a 5 mL clear low density polyethylene bottle with a clear polyethylene dropper tip, a turquoise high density polyethylene screw cap, and a tamper-evident clear low density polyethylene overcap. 2.5 mL fill, 50 mcg/mL (0.005%): Package of 1 bottle: NDC 0013-8303-04 Pfizer Manufacturing Belgium NV, Puurs, Belgium |
Drugs
| Drug | Countries | |
|---|---|---|
| XALATAN | Australia, Brazil, Canada, Cyprus, Germany, Ecuador, Estonia, Spain, Finland, France, Hong Kong, Croatia, Ireland, Israel, Italy, Japan, Lithuania, Malta, Mexico, Nigeria, Netherlands, Poland, Romania, Singapore, Tunisia, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.