ZITHROMAX Film-coated tablet / Powder for suspension Ref.[10631] Active ingredients: Azithromycin
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
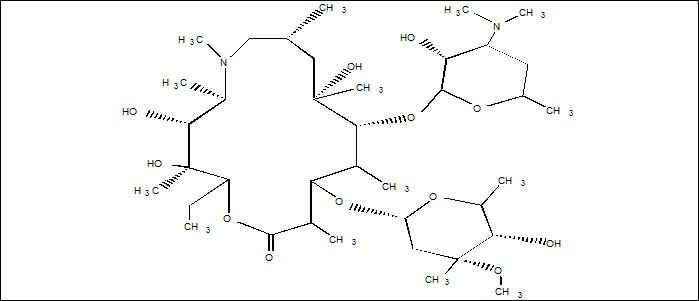
ZITHROMAX (azithromycin tablets and azithromycin for oral suspension) contain the active ingredient azithromycin, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)13[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl) oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C38H72N2O12, and its molecular weight is 749.00.
Azithromycin has the following structural formula:
Azithromycin, as the dihydrate, is a white crystalline powder with a molecular formula of C38H72N2O12∙2H2O and a molecular weight of 785.0.
ZITHROMAX is supplied as tablets containing azithromycin dihydrate equivalent to either 250 mg or 500 mg azithromycin and the following inactive ingredients: dibasic calcium phosphate anhydrous, pregelatinized starch, sodium croscarmellose, magnesium stearate, sodium lauryl sulfate, hypromellose, lactose, titanium dioxide, triacetin, and D&C Red #30 aluminum lake.
ZITHROMAX for oral suspension is supplied in bottles containing azithromycin dihydrate powder equivalent to 300 mg, 600 mg, 900 mg, or 1200 mg azithromycin per bottle and the following inactive ingredients: sucrose; sodium phosphate, tribasic, anhydrous; hydroxypropyl cellulose; xanthan gum; FD&C Red #40; and spray dried artificial cherry, creme de vanilla, and banana flavors. After constitution, each 5 mL of suspension contains 100 mg or 200 mg of azithromycin.
| Dosage Forms and Strengths |
|---|
|
ZITHROMAX 250 mg tablets are supplied as pink modified capsular shaped, engraved, film-coated tablets containing azithromycin dihydrate equivalent to 250 mg of azithromycin. ZITHROMAX 250 mg tablets are engraved with “PFIZER” on one side and “306” on the other, or “Pfizer” on one side and “ZTM 250” on the other. These are packaged in bottles and blister cards of 6 tablets (Z-PAKS). ZITHROMAX 500 mg tablets are supplied as pink modified capsular shaped, engraved, film-coated tablets containing azithromycin dihydrate equivalent to 500 mg of azithromycin. ZITHROMAX 500 mg tablets are engraved with “Pfizer” on one side and “ZTM500” on the other. These are packaged in bottles and blister cards of 3 tablets (TRI-PAKS). ZITHROMAX for oral suspension after constitution contains a flavored suspension. ZITHROMAX for oral suspension is supplied to provide 100 mg/5 mL or 200 mg/5 mL suspension in bottles. |
| How Supplied | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ZITHROMAX is supplied in the following strengths and package configurations:
ZITHROMAX tablets should be stored between 15° to 30°C (59° to 86°F). ZITHROMAX for oral suspension after constitution contains a flavored suspension. ZITHROMAX for oral suspension is supplied to provide 100 mg/5 mL or 200 mg/5 mL suspension in bottles as follows:
[see Dosage and Administration (2)] for constitution instructions with each bottle type. |
Drugs
| Drug | Countries | |
|---|---|---|
| ZITHROMAX | Austria, Australia, Canada, Cyprus, Germany, Finland, France, Hong Kong, Ireland, Israel, Malta, Nigeria, Netherlands, New Zealand, Singapore, Tunisia, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.