CYSTADANE Powder for oral solution Ref.[10796] Active ingredients: Betaine
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
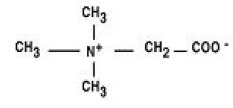
CYSTADANE (betaine anhydrous for oral solution) is an agent for the treatment of homocystinuria. It contains no ingredients other than anhydrous betaine. CYSTADANE is a white, granular, hygroscopic powder, which is diluted in water and administered orally. The chemical name of betaine anhydrous powder is trimethylglycine. It has a molecular weight of 117.15.
The structural formula is:
| Dosage Forms and Strengths |
|---|
|
CYSTADANE is a white, granular, hygroscopic powder for oral solution available in bottles containing 180 grams of betaine anhydrous. |
| How Supplied |
|---|
|
CYSTADANE is available in plastic bottles containing 180 grams of betaine anhydrous as a white, granular, hygroscopic powder. Each bottle is equipped with a plastic child-resistant cap and is supplied with a polypropylene measuring scoop. One level scoop (1.7 mL) is equal to 1 gram of betaine anhydrous powder. NDC 52276-400-01 180 g/bottle Supplied by: Recordati Rare Diseases, Puteaux, France Licensed to and Distributed by: Recordati Rare Diseases Inc., Lebanon, NJ 08833 U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| CYSTADANE | Austria, Australia, Brazil, Canada, Estonia, Spain, Finland, France, Croatia, Ireland, Israel, Japan, Lithuania, Netherlands, New Zealand, Poland, Romania, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.