DIACOMIT Capsule / Powder for suspension Ref.[10826] Active ingredients: Stiripentol
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
The mechanism by which DIACOMIT exerts its anticonvulsant effect in humans is unknown. Possible mechanisms of action include direct effects mediated through the gamma-aminobutyric acid (GABA)A receptor and indirect effects involving inhibition of cytochrome P450 activity with resulting increase in blood levels of clobazam and its active metabolite.
12.2. Pharmacodynamics
There are no relevant data on the pharmacodynamic effects of DIACOMIT.
12.3. Pharmacokinetics
The following pharmacokinetic properties of stiripentol have been found in studies in adult healthy volunteers and adult patients. Systemic exposure of stiripentol increases in a greater than dose proportional manner from 500 mg to 2000 mg.
Absorption
The median time to stiripentol peak plasma concentration is 2 to 3 hours.
Distribution
Protein binding of stiripentol is 99%.
Elimination
The elimination half-life of stiripentol ranges from 4.5 to 13 hours, increasing with doses of 500 mg, 1000 mg and 2000 mg.
Metabolism
On the basis of in vitro studies, the main liver cytochrome P450 (CYP) isoenzymes involved in metabolism are considered to be CYP1A2, CYP2C19, and CYP3A4.
h3.Specific Populations
The effect of age (≥65 years), race, renal and hepatic impairment on stiripentol pharmacokinetics is unknown [see Use in Specific Populations (8.5, 8.6, 8.7)]. Sex does not have a clinically significant effect on the pharmacokinetics of DIACOMIT.
Pediatric Patients
In a study of children (median age 7.3 years) with Dravet syndrome treated with DIACOMIT, valproate, and clobazam, the apparent clearance and volume of distribution of stiripentol were related to body weight. Elimination half-life increased from 8.5 hr (for 10 kg) to 23.5 hr (for 60 kg).
Drug Interaction Studies
In Vitro Studies
The metabolic pathway for stiripentol has not been clearly elucidated. Stiripentol is a substrate of several CYP enzymes, including CYP1A2, CYP2C19, and CYP3A4. Stiripentol inhibits and induces CYP1A2, CYP2B6, and CYP3A4. Stiripentol also inhibits CYP2C8, CYP2C19, and drug transporters, including P-gp and BCRP, at clinically relevant concentrations [see Drug Interactions (7.1)].
Clinical Studies
Antiepileptic drugs: Co-administration of clobazam with stiripentol increased concentrations of clobazam by approximately 2-fold and norclobazam (clobazam active metabolite) by 5-fold [see Drug Interactions (7.1)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In mice, oral administration of stiripentol (0, 60, 200, or 600 mg/kg/day) for 78 weeks increased the incidences of liver tumors (hepatocellular adenoma and carcinoma) at the mid and high dose. The dose not associated with an increase in liver tumors (60 mg/kg/day) is less than the recommended human dose (RHD) of 50 mg/kg/day, based on body surface area (mg/m2). In rats, oral administration of stiripentol at doses of up to 800 mg/kg/day (approximately 2.5 times the RHD on a mg/m 2 basis) for 102 weeks did not result in an increase in tumors.
Mutagenesis
Stiripentol was negative for genotoxicity in in vitro (Ames, HPRT gene mutation in V79 Chinese hamster cells, and chromosomal aberration in human lymphocytes) and in vivo (mouse bone marrow micronucleus) assays. Stiripentol was clastogenic in CHO cells in vitro, but only at cytotoxic concentrations.
Impairment of Fertility
Oral administration of stiripentol (0, 50, 200, or 800 mg/kg/day) to male and female rats prior to and throughout mating and continuing in females throughout organogenesis produced no adverse effects on fertility. The highest dose tested is approximately 2.5 times the RHD on mg/m2 basis.
14. Clinical Studies
The effectiveness of DIACOMIT for the treatment of seizures associated with Dravet syndrome was established in 2 multicenter placebo-controlled double-blind randomized studies (Study 1 and Study 2), conducted according to similar protocols. To be enrolled in either study, patients were required to be 3 years to less than 18 years of age, to have Dravet syndrome (ILAE classification of epilepsy, 1989), and to be inadequately controlled on clobazam and valproate, with at least 4 generalized clonic or tonic-clonic seizures per month despite optimized therapy.
Eligible patients were enrolled in a 1-month baseline period during which they continued to receive their optimized antiepileptic treatment. Following this 1-month baseline, patients were randomly allocated to receive either DIACOMIT (fixed dose of 50 mg/kg/day in divided doses with no dose titration) or placebo, added to their treatment with clobazam and valproate. Duration of double-blind treatment was 2 months. The frequency of generalized clonic or tonic-clonic seizures during the study was recorded by patients and/or their caregivers, using a diary. Although patients with Dravet syndrome have several different types of seizures, only generalized clonic or tonic-clonic seizures were recorded, as other seizure types can be difficult to recognize by patients and/or their caregivers as seizures.
The primary efficacy endpoint for both studies was the responder rate. A responder was defined as a patient who experienced a greater than 50% decrease in the frequency (per 30 days) of generalized clonic or tonic-clonic seizures during the double-blind treatment period compared to the 4-week baseline period (i.e., placebo run-in). The mean change from baseline in frequency of generalized clonic or tonic clonic seizures was also evaluated.
In Study 1 (n=41), 21 patients were randomized to DIACOMIT, and 20 patients to placebo. In Study 2 (n=23), 12 patients were randomized to DIACOMIT, and 11 patients to placebo. In both studies, the demographic and baseline clinical characteristics were similar between the treatment groups.
Table 4 summarizes the results of the primary endpoint for DIACOMIT in each study.
Table 4. Efficacy Results in the Intent-to-Treat Population in Study 1 and Study 2:
| Study 1 N=41 | Study 2 N=23 | |||
|---|---|---|---|---|
| DIACOMIT N=21 | Placebo N=20 | DIACOMIT N=12 | Placebo N=11 | |
| Responder Analysisa | ||||
| No of responders/total (Responder Rate) [95% CI] | 15/21 (71%) [52% – 91%] | 1/20 (5%) [0.0% – 15%] | 8/12 (67%) [40% – 93%] | 1/11 (9.1%) [0.0% – 26%] |
| p-valueb | <0.0001 | 0.0094e | ||
| Percentage Change from Baseline in Seizure Frequencyc | ||||
| n Mean ± SD Median Min – Max | 20 -69% ± 42% -91% -100% – 28% | 16 7.6% ± 38% 7.4% -75% – 65% | 11 -74% ± 27% -81% -100% – -33% | 9 -13% ± 62% -27% -87% – 140% |
| p-valued | 0.0002 | 0.0056e | ||
a Responder is defined as a patient with a greater than 50% decrease in frequency of generalized tonic-clonic or clonic seizures
b Fisher Exact Test
c Frequency of generalized tonic-clonic or clonic seizures during month 2
d Wilcoxon Test with two-sided t-approximation
e Nominal p value, as Study 2 was stopped early
CI=confidence interval; SD=standard deviation.
In both studies, the responder rate (primary efficacy endpoint) was significantly greater for DIACOMIT than for placebo. DIACOMIT was also superior to placebo for the reduction in mean frequency of generalized clonic or tonic-clonic seizures. In Study 1 and Study 2, respectively 43% and 25% of patients reported no generalized clonic or tonic-clonic seizure for the duration of the study.
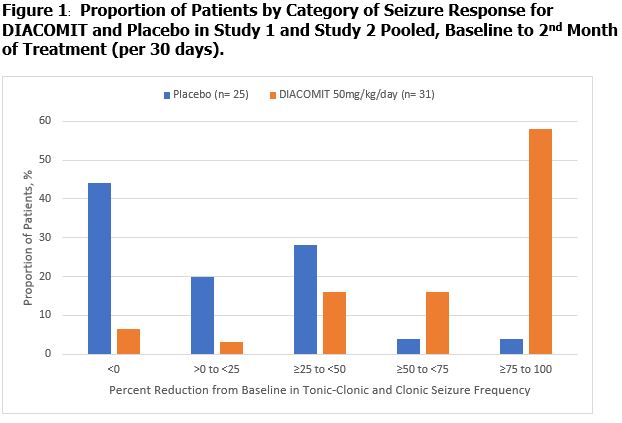
Figure 1 displays the percentage of patients by category of percent reduction in tonic-clonic and clonic seizure frequency during month 2 of the treatment period compared to baseline (per 30 days) in Study 1 and Study 2 (pooled).
The effectiveness of DIACOMIT for the treatment of seizures associated with Dravet syndrome in patients 2 years of age to less than 3 years of age was extrapolated from the demonstration of effectiveness in patients 3 years to less than 18 years of age in Study 1 and Study 2.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.