QUININE SULFATE Capsule Ref.[108424] Active ingredients: Quinine
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
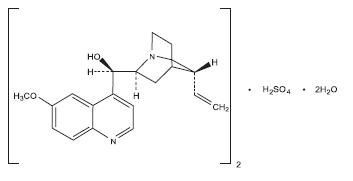
Quinine sulfate is a cinchona alkaloid chemically described as cinchonan-9-ol, 6'methoxy, (8α, 9R)-, sulfate (2:1) (salt), dihydrate with a molecular formula of (C20H24N2O2)•H2SO4•2H2O and a molecular weight of 782.94.
The structural formula of quinine sulfate is:
Quinine sulfate occurs as a white, crystalline powder that darkens on exposure to light. It is odorless and has a persistent very bitter taste. It is only slightly soluble in water, alcohol, chloroform, and ether.
Quinine sulfate capsules USP are supplied for oral administration as capsules containing 324 mg of the active ingredient quinine sulfate USP, equivalent to 269 mg free base. Inactive ingredients: black iron oxide, corn starch, gelatin, magnesium stearate, potassium hydroxide, propylene glycol shellac, sodium lauryl sulfate, and talc.
| Dosage Forms and Strengths |
|---|
|
324 mg size “0” capsules with clear transparent cap and clear transparent body imprinted with “LU” on cap and “Y51” on body in black ink, containing white to off white powder. |
| How Supplied |
|---|
|
Quinine sulfate capsules USP, 324 mg are available as size “0” capsules with clear transparent cap and clear transparent body imprinted with “LU” on cap and “Y51” on body in black ink, containing white to off white powder: Bottles of 30 NDC 68180-560-06 Manufactured for: Lupin Pharmaceuticals, Inc., Baltimore, Maryland 21202, United States Manufactured by: Lupin Limited, Goa 403 722, INDIA |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.