FLUDEOXYGLUCOSE F18 Solution for injection Ref.[11029] Active ingredients: Fludeoxyglucose ¹⁸F
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
11.1 Chemical Characteristics
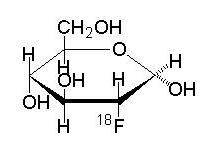
Fludeoxyglucose F18 Injection USP is a positron emitting radiopharmaceutical that is used for diagnostic purposes in conjunction with positron emission tomography (PET) imaging. The active ingredient 2-deoxy-2-[18F]fluoro-D-glucose has the molecular formula of C6H1118FO5 with a molecular weight oF181.26, and has the following chemical structure:
Fludeoxyglucose F18 Injection is provided as a ready to use sterile, pyrogen free, clear, colorless citrate buffered solution. Each mL contains between 0.740 to 11.1GBq (20.0-300 mCi) of 2-deoxy-2-[18F]fluoro-D-glucose at the EOS, 4.5 mg of sodium chloride in citrate buffer. The pH of the solution is between 4.5 and 7.5. The solution is packaged in a multiple-dose glass vial and does not contain any preservative.
11.2 Physical Characteristics
Fluorine F18 has a physical half-life of 109.7 minutes and decays to Oxygen O-18 (stable) by positron decay. The principal photons useful for imaging are the dual 511 keV "annihilation" gamma photons that are produced and emitted simultaneously in opposite directions when the positron interacts with an electron (Table 2).
Table 2. Principal Radiation Emission Data for Fluorine F18:
| Radiation/Emission | % Per Disintegration | Mean Energy |
|---|---|---|
| Positron(β+) | 96.73 | 249.8 keV |
| Gamma(±)* | 193.46 | 511.0 keV |
* Produced by positron annihilation
From: Kocher, D.C. Radioactive Decay Tables DOE/TIC-I 1026, 89 (1981)
The specific gamma ray constant (point source air kerma coefficient) for fluorine F18 is 5.7 R/hr/mCi (1.35 x 10-6 Gy/hr/kBq) at 1 cm. The half-value layer (HVL) for the 511 keV photons is 4 mm lead (Pb). The range of attenuation coefficients for this radionuclide as a function of lead shield thickness is shown in Table 3. For example, the interposition of an 8 mm thickness of Pb, with a coefficient of attenuation of 0.25, will decrease the external radiation by 75%.
Table 3. Radiation Attenuation of 511 keV Photons by lead (Pb) shielding:
| Shield thickness (Pb) mm | Coefficient of attenuation |
|---|---|
| 0 | 0.00 |
| 4 | 0.50 |
| 8 | 0.25 |
| 13 | 0.10 |
| 26 | 0.01 |
| 39 | 0.001 |
| 52 | 0.0001 |
For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 4.
Table 4. Physical Decay Chart for Fluorine F18:
| Minutes | Fraction Remaining |
|---|---|
| 0* | 1.000 |
| 15 | 0.909 |
| 30 | 0.826 |
| 60 | 0.683 |
| 110 | 0.500 |
| 220 | 0.250 |
* calibration time
| Dosage Forms and Strengths |
|---|
|
Multiple-dose glass vial containing 0.74-11.1GBq (20-300 mCi/mL) of Fludeoxyglucose F18 Injection USP and 4.5 mg of sodium chloride in citrate buffer (approximately 24-30 mL volume) for intravenous administration. |
| How Supplied |
|---|
|
Fludeoxyglucose F18 Injection USP is supplied in a multi-dose, capped glass vial containing between 0.740–11.1GBq/mL (20-300 mCi/mL), of no carrier added 2-deoxy-2-[F18]fluoro-D-glucose, at end of synthesis, in approximately 24-30 mL. The contents of each vial are sterile, pyrogen-free and preservative-free. 30 mL Vial: NDC 51760-001-30 This radiopharmaceutical is licensed by the Nuclear Regulatory Commission, for distribution to entities licensed pursuant to 10 CFR 35.200 or under the equivalent licenses of an Agreement State or Licensing State. Manufactured and Distributed by: Essential Isotopes LLC, 1513 Research Park Dr, Columbia, Missouri 65211 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.