FLUORODOPA F18 Solution for injection Ref.[11031] Active ingredients: Fluorodopa ¹⁸F
Source: FDA, National Drug Code (US) Revision Year: 2021
1. Indications and Usage
Fluorodopa F18 Injection is indicated for use in positron emission tomography (PET) to visualize dopaminergic nerve terminals in the striatum for the evaluation of adult patients with suspected Parkinsonian syndromes (PS). Fluorodopa F18 PET is an adjunct to other diagnostic evaluations.
2. Dosage and Administration
2.1 Radiation Safety - Drug Handling
Handle Fluorodopa F18 Injection with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions (5.1)]. Use waterproof gloves, effective shielding and appropriate safety measures to avoid unnecessary radiation exposure to the patient, occupational workers, clinical personnel, and other persons.
2.2 Recommended Dosage and Administration
Recommended Dose:
- The recommended dose for adults is 185 megabecquerels (MBq) [5 millicuries (mCi)] administered as an intravenous injection infused over 1 minute.
- Minimize the dose of Fluorodopa F18 Injection consistent with the objectives of the procedure and the nature of the imaging cameras employed.
Administration:
- Use aseptic techniques and radiation shielding during all operations involved in the manipulation and administration of Fluorodopa F18 Injection.
- Calculate the necessary volume to administer based on calibration time and dose.
- Inspect Fluorodopa F18 Injection visually and do not use the drug if the solution contains particulate matter or is discolored.
- Measure the patient dose immediately prior to administration in a dose calibrator.
- Dispose of unused drug in compliance with applicable regulations.
2.3 Patient Preparation
Hydration:
- Patients must have nothing to eat or drink, except water for 4 hours before administration
- To minimize the radiation absorbed dose to the bladder, instruct patient to:
- hydrate 4 hours prior to the administration of Fluorodopa F18 Injection and continue hydration after the study.
- void 70 minutes after administration of Fluorodopa F18 Injection and as frequently thereafter as possible for the next 12 hours.
Pre-medication and Medication Withdrawal:
- Carbidopa blocks systemic/peripheral decarboxylation of Fluorodopa F18 Injection to increase uptake in the brain. Administer 150 mg of Carbidopa orally at least 60 minutes (and no longer than 120 minutes) prior to the administration of Fluorodopa F18 Injection.
- Instruct the patient to discontinue medications for the treatment of Parkinson's disease 12 hours prior to administration of Fluorodopa F18 Injection [see Drug Interactions (7)].
2.4 Radiation Dosimetry
The estimated human absorbed radiation dose for 10 human subjects (8 men and 2 women; mean age 50±8.8 yr) from intravenous administration of Fluorodopa F18 Injection is shown in Table 1. In subjects who voided at 40 min post-injection, the radiation absorbed dose to the bladder wall was 50% lower than the radiation absorbed dose in subjects who voided at 2 hours post-injection. The identified critical organ is the urinary bladder.
Table 1. Estimated Absorbed Radiation Dose (mGy/MBq) for Adult Patients after Intravenous Administration of Fluorodopa F18 Injectiona:
| Organ | Absorbed Dose per unit activity (mGy/MBq) |
|---|---|
| Urinary bladder wall | 0.30 |
| Heart wall | 0.01 |
| Pancreas | 0.01 |
| Spleen | 0.01 |
| Lungs | 0.01 |
| Kidneys | 0.03 |
| Ovaries | 0.02 |
| Uterus | 0.03 |
| Lower Large Intestine wall | 0.02 |
| Liver | 0.01 |
| Gallbladder wall | 0.01 |
| Small Intestine | 0.01 |
| Upper Large Intestine wall | 0.01 |
| Stomach wall | 0.01 |
| Adrenals | 0.01 |
| Testes | 0.01 |
| Red marrow | 0.01 |
| Thymus | 0.01 |
| Thyroid | 0.01 |
| Muscle | 0.01 |
| Bone surfaces | 0.01 |
| Breast | 0.01 |
| Skin | 0.01 |
| Brain | 0.01 |
| Remaining organs | 0.01 |
| Effective dose (mSv/MBq) | 0.03 |
a ICRP Publication 128, Radiation Dose to Patients from Radiopharmaceuticals, Annals of the ICRP, Vol. 44, No. 2S, 2015.
2.5 Imaging Guidelines
- Instruct the patient to void immediately before imaging, 70 minutes post administration.
- Start imaging at about 80 minutes post administration (with a 9 second CT scan for attenuation correction) followed by 3D PET scan from 80 to 100 minutes post administration.
2.6 Image Interpretation
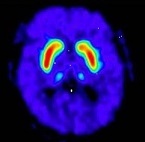
Fluorodopa F18 PET scans are interpreted visually, based upon the appearance and shape of the putamen and caudate of the striatum. Optimum presentation of the reconstructed images for visual interpretation is transaxial slices parallel to the anterior commissure-posterior commissure (AC-PC) line. Determination of whether an image is negative or positive is made by assessing the shape and intensity of the striatal signal (see Figure 1 and 2). Image interpretation does not involve integration of the image with clinical signs and/or symptoms.
Negative Scans: A full crescent shaped putamen and caudate image (see Figure 1). FDOPA uptake is clearly delineated from the background activity in the brain. It is bilaterally symmetrical and of uniform thickness in both caudate and putamen (comma- or crescent-shaped).
Figure 1. Negative Scan:
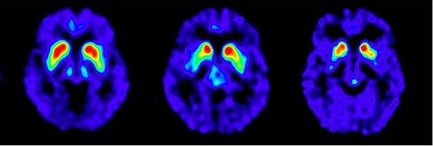
Positive Scans: A reduction in the size and shape of putamen (unilaterally or bilaterally) or both putamen and caudate (unilaterally or bilaterally). All of the following are considered positive:
* FDOPA uptake is asymmetric in the putamen; normal on one side but reduced on the contralateral side with respect to the background, especially in the posterior part. Caudate uptake is symmetrical on both sides and clearly delineated from the background (see Figure 2, A).
* FDOPA uptake is bilaterally reduced in the putamen (see Figure 2, B).
* FDOPA uptake is bilaterally reduced in the putamen and in the caudate nuclei (see Figure 2, C).
Figure 2. Positive Scans:
A – B – C
16.2. Storage and Handling
Storage
Store the Fluorodopa F18 Injection vial upright in a lead shielded container at 25°C (77°F); excursions permitted between 15ºC to 30°C (59ºF to 86°F). Avoid direct light.
The expiration date and time are provided on the container label. Use Fluorodopa F18 Injection within 8 hours from the time of the end of synthesis (EOS).
Handling
This radiopharmaceutical is for distribution and use by persons licensed authorized by the U.S. Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State. Store and dispose of Fluorodopa F18 in compliance with the appropriate regulations of the government agency authorized to license the use of this radionuclide.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.