Velcade 3.5 mg powder for solution for injection Ref.[2571] Active ingredients: Bortezomib
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2012 Publisher: JANSSEN-CILAG INTERNATIONAL NV Turnhoutseweg 30 B-2340 Beerse Belgium
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, other antineoplastic agents
ATC code: L01XX32
Mechanism of action
Bortezomib is a proteasome inhibitor. It is specifically designed to inhibit the chymotrypsin-like activity of the 26S proteasome in mammalian cells. The 26S proteasome is a large protein complex that degrades ubiquitinated proteins. The ubiquitin-proteasome pathway plays an essential role in regulating the turnover of specific proteins, thereby maintaining homeostasis within cells. Inhibition of the 26S proteasome prevents this targeted proteolysis and affects multiple signalling cascades within the cell, ultimately resulting in cancer cell death.
Bortezomib is highly selective for the proteasome. At 10 μM concentrations, bortezomib does not inhibit any of a wide variety of receptors and proteases screened and is more than 1500-fold more selective for the proteasome than for its next preferable enzyme. The kinetics of proteasome inhibition were evaluated in vitro, and bortezomib was shown to dissociate from the proteasome with a t½ of 20 minutes, thus demonstrating that proteasome inhibition by bortezomib is reversible.
Bortezomib mediated proteasome inhibition affects cancer cells in a number of ways, including, but not limited to, altering regulatory proteins, which control cell cycle progression and nuclear factor kappa B (NF-kB) activation. Inhibition of the proteasome results in cell cycle arrest and apoptosis. NF-kB is a transcription factor whose activation is required for many aspects of tumourigenesis, including cell growth and survival, angiogenesis, cell-cell interactions, and metastasis. In myeloma, bortezomib affects the ability of myeloma cells to interact with the bone marrow microenvironment.
Experiments have demonstrated that bortezomib is cytotoxic to a variety of cancer cell types and that cancer cells are more sensitive to the pro-apoptotic effects of proteasome inhibition than normal cells. Bortezomib causes reduction of tumour growth in vivo in many preclinical tumour models, including multiple myeloma.
Data from in vitro, ex-vivo, and animal models with bortezomib suggest that it increases osteoblast differentiation and activity and inhibits osteoclast function. These effects have been observed in patients with multiple myeloma affected by an advanced osteolytic disease and treated with bortezomib.
Clinical efficacy in previously untreated multiple myeloma
A prospective Phase III, international, randomised (1:1), open-label clinical study (VISTA) of 682 patients was conducted to determine whether VELCADE (1.3 mg/m² injected intravenously) in combination with melphalan (9 mg/m²) and prednisone (60 mg/m²) resulted in improvement in time to progression (TTP) when compared to melphalan (9 mg/m²) and prednisone (60 mg/m²) in patients with previously untreated multiple myeloma. Treatment was administered for a maximum of 9 cycles (approximately 54 weeks) and was discontinued early for disease progression or unacceptable toxicity. The median age of the patients in the study was 71 years, 50% were male, 88% were Caucasian and the median Karnofsky performance status score for the patients was 80. Patients had IgG/IgA/Light chain myeloma in 63%/25%/8% instances, a median hemoglobin of 105 g/L, and a median platelet count of 221.5 x 109/l. Similar proportions of patients had creatinine clearance ≤ 30 ml/min (3% in each arm).
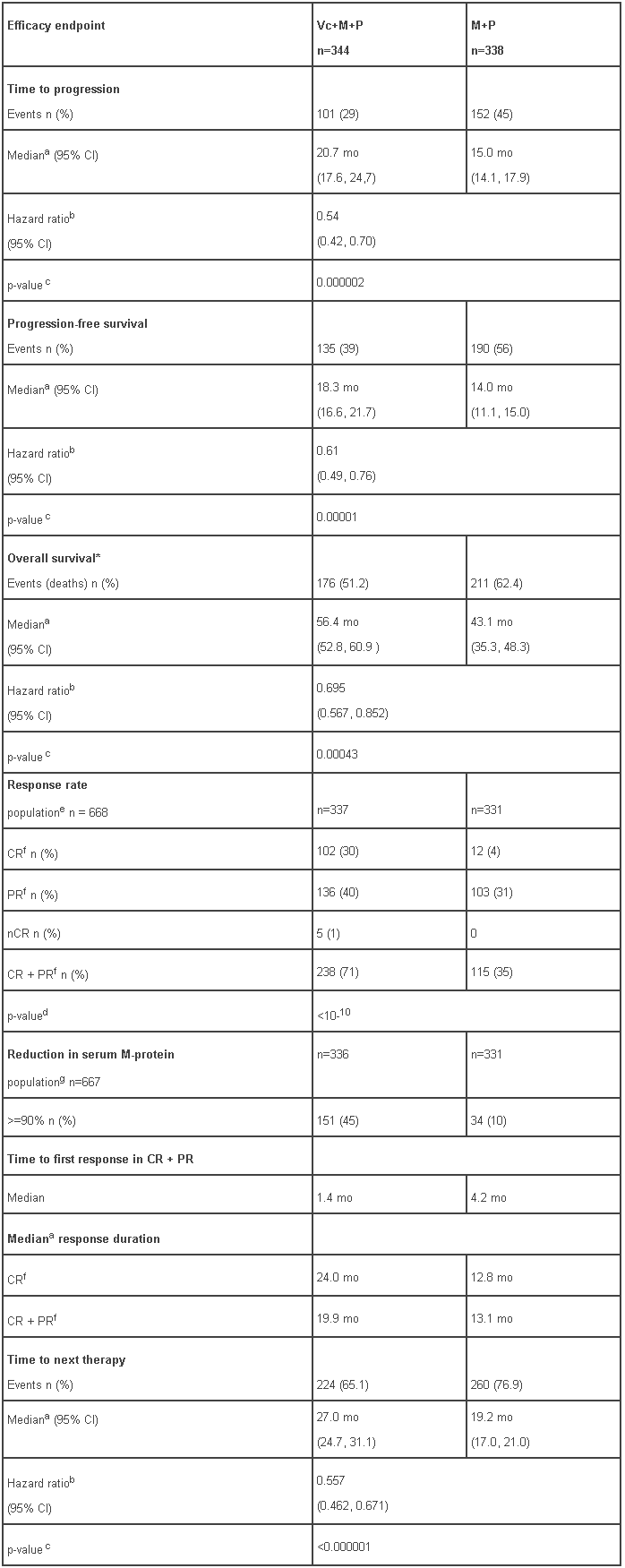
At the time of a pre-specified interim analysis, the primary endpoint, time to progression, was met and patients in the M+P arm were offered Vc+M+P treatment. Median follow-up was 16.3 months. The final survival update was performed with a median duration of follow-up of 60.1 months. A statistically significant survival benefit in favour of the Vc+M+P treatment group was observed (HR=0.695; p=0.00043) despite subsequent therapies including VELCADE-based regimens. Median survival for the Vc+M+P treatment group was 56.4 months compared to 43.1 for the M+P treatment group. Efficacy results are presented in Table 6:
Table 6 - Efficacy results following the final survival update to VISTA study:
a Kaplan-Meier estimate.
b Hazard ratio estimate is based on a Cox proportional-hazard model adjusted for stratification factors: β2-microglobulin, albumin, and region. A hazard ratio less than 1 indicates an advantage for VMP
c Nominal p-value based on the stratified log-rank test adjusted for stratification factors: β2-microglobulin, albumin, and region
d p-value for Response Rate (CR + PR) from the Cochran-Mantel-Haenszel chi-square test adjusted for the stratification factors
e Response population includes patients who had measurable disease at baseline
f CR = Complete Response; PR = Partial Response. EBMT criteria
g All randomized patients with secretory disease
* Survival update based on a median duration of follow-up at 60.1 months
mo: months
CI = Confidence Interval
Clinical efficacy in relapsed or refractory multiple myeloma
The safety and efficacy of VELCADE (injected intravenously) were evaluated in 2 studies at the recommended dose of 1.3 mg/m²: a Phase III randomized, comparative study (APEX), versus dexamethasone (Dex), of 669 patients with relapsed or refractory multiple myeloma who had received 1-3 prior lines of therapy, and a Phase II single-arm study of 202 patients with relapsed and refractory multiple myeloma, who had received at least 2 prior lines of treatment and who were progressing on their most recent treatment.
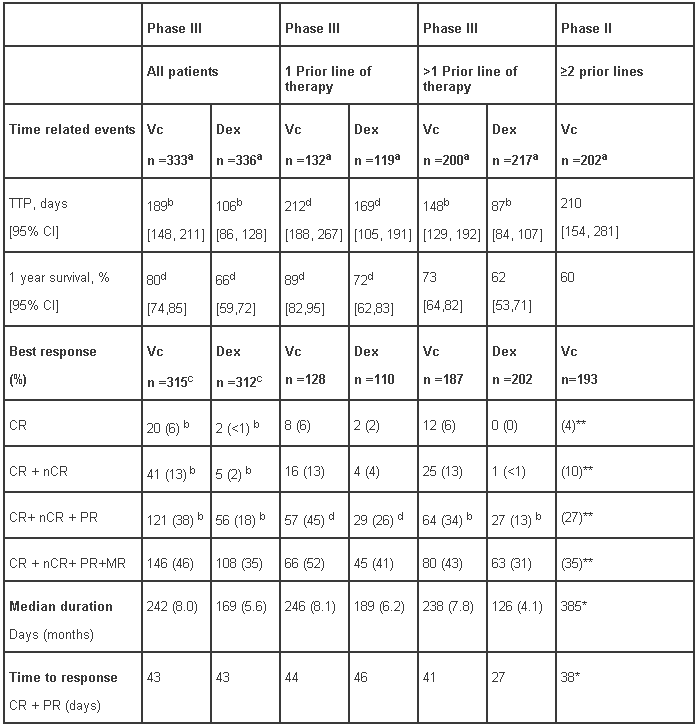
In the Phase III study, treatment with VELCADE led to a significantly longer time to progression, a significantly prolonged survival and a significantly higher response rate, compared to treatment with dexamethasone (see Table 7), in all patients as well as in patients who have received 1 prior line of therapy. As a result of a pre-planned interim analysis, the dexamethasone arm was halted at the recommendation of the data monitoring committee and all patients randomised to dexamethasone were then offered VELCADE, regardless of disease status. Due to this early crossover, the median duration of follow-up for surviving patients is 8.3 months. Both in patients who were refractory to their last prior therapy and those who were not refractory, overall survival was significantly longer and response rate was significantly higher on the VELCADE arm.
Of the 669 patients enrolled, 245 (37%) were 65 years of age or older. Response parameters as well as TTP remained significantly better for VELCADE independently of age. Regardless of β2- microglobulin levels at baseline, all efficacy parameters (time to progression and overall survival, as well as response rate) were significantly improved on the VELCADE arm.
In the refractory population of the Phase II study, responses were determined by an independent review committee and the response criteria were those of the European Bone Marrow Transplant Group. The median survival of all patients enrolled was 17 months (range <1 to 36+ months). This survival was greater than the six-to-nine month median survival anticipated by consultant clinical investigators for a similar patient population. By multivariate analysis, the response rate was independent of myeloma type, performance status, chromosome 13 deletion status, or the number or type of previous therapies. Patients who had received 2 to 3 prior therapeutic regimens had a response rate of 32% (10/32) and patients who received greater than 7 prior therapeutic regimens had a response rate of 31% (21/67).
Table 7 - Summary of disease outcomes from the Phase III (APEX) and Phase II studies:
a Intent to Treat (ITT) population
b p-value from the stratified log-rank test; analysis by line of therapy excludes stratification for therapeutic history; p<0.0001
c Response population includes patients who had measurable disease at baseline and received at least 1 dose of study medicinal product.
d p-value from the Cochran-Mantel-Haenszel chi-square test adjusted for the stratification factors; analysis by line of therapy excludes stratification for therapeutic history
* CR+PR+MR
** CR=CR, (IF-); nCR=CR (IF+)
NA = not applicable, NE = not estimated
TTP-Time to Progression
CI = Confidence Interval
Vc = VELCADE; Dex = dexamethasone
CR = Complete Response; nCR- near Complete response
PR = Partial Response; MR-Minimal response
In the Phase II study, patients who did not obtain an optimal response to therapy with VELCADE alone were able to receive high-dose dexamethasone in conjunction with VELCADE.The protocol allowed patients to receive dexamethasone if they had had a less than optimal response to VELCADE alone. A total of 74 evaluable patients were administered dexamethasone in combination with VELCADE. Eighteen percent of patients achieved, or had an improved response (MR (11%) or PR (7%) with combination treatment.
Clinical efficacy with subcutaneous administration of VELCADE in patients with relapsed/refractory multiple myeloma
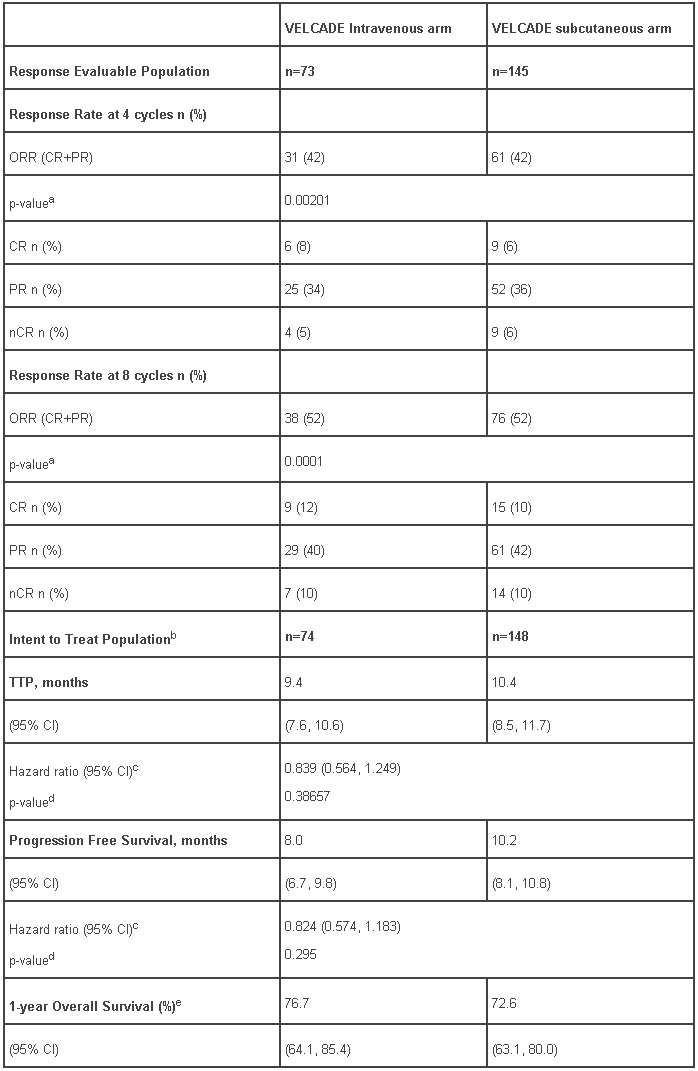
An open label, randomized, Phase III non-inferiority study compared the efficacy and safety of the subcutaneous administration of VELCADE versus the intravenous administration. This study included 222 patients with relapsed/refractory multiple myeloma, who were randomized in a 2:1 ratio to receive 1.3 mg/m² of VELCADE by either the subcutaneous or intravenous route for 8 cycles. Patients who did not obtain an optimal response (less than Complete Response [CR]) to therapy with VELCADE alone after 4 cycles were allowed to receive dexamethasone 20 mg daily on the day of and after VELCADE administration. Patients with baseline grade ≥ 2 peripheral neuropathy or platelet counts <50,000/µl were excluded. A total of 218 patients were evaluable for response.
This study met its primary objective of non-inferiority for response rate (CR+PR) after 4 cycles of single agent VELCADE for both the subcutaneous and intravenous routes, 42% in both groups. In addition, secondary response-related and time to event related efficacy endpoints showed consistent results for subcutaneous and intravenous administration (Table 8).
Table 8 - Summary of efficacy analyses comparing subcutaneous and intravenous administrations of VELCADE:
a p-value is for the non-inferiority hypothesis that the SC arm retains at least 60% of the response rate in the IV arm.
b 222 subjects were enrolled into the study; 221 subjects were treated with VELCADE
c Hazards ratio estimate is based on a Cox model adjusted for stratification factors: ISS staging and number of prior lines.
d Log rank test adjusted for stratification factors: ISS staging and number of prior lines.
e Median duration of follow up is 11.8 months
Patients with previously treated light-chain (AL) Amyloidosis
An open label non randomised Phase I/II study was conducted to determine the safety and efficacy of VELCADE in patients with previously treated light-chain (AL) Amyloidosis. No new safety concerns were observed during the study, and in particular VELCADE did not exacerbate target organ damage (heart, kidney and liver). In an exploratory efficacy analysis, a 67.3% response rate (including a 28.6% CR rate) as measured by hematologic response (M-protein) was reported in 49 evaluable patients treated with the maximum allowed doses of 1.6 mg/m² weekly and 1.3 mg/m² twice-weekly. For these dose cohorts, the combined 1-year survival rate was 88.1%.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with VELCADE in all subsets of the paediatric population in multiple myeloma (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Following intravenous bolus administration of a 1.0 mg/m² and 1.3 mg/m² dose to 11 patients with multiple myeloma and creatinine clearance values greater than 50 ml/min, the mean first-dose maximum plasma concentrations of bortezomib were 57 and 112 ng/ml, respectively. In subsequent doses, mean maximum observed plasma concentrations ranged from 67 to 106 ng/ml for the 1.0 mg/m² dose and 89 to 120 ng/ml for the 1.3 mg/m² dose.
Following an intravenous bolus or subcutaneous injection of a 1.3 mg/m² dose to patients with multiple myeloma (n=14 in the intravenous group, n=17 in the subcutaneous group), the total systemic exposure after repeat dose administration (AUClast) was equivalent for subcutaneous and intravenous administrations. The Cmax after subcutaneous administration (20.4 ng/ml) was lower than intravenous (223 ng/ml). The AUClast geometric mean ratio was 0.99 and 90% confidence intervals were 80.18% - 122.80%.
Distribution
The mean distribution volume (Vd) of bortezomib ranged from 1659 l to 3294 l following single- or repeated-dose intravenous administration of 1.0 mg/m² or 1.3 mg/m² to patients with multiple myeloma. This suggests that bortezomib distributes widely to peripheral tissues. Over a bortezomib concentration range of 0.01 to 1.0 μg/ml, the in vitro protein binding averaged 82.9% in human plasma. The fraction of bortezomib bound to plasma proteins was not concentration-dependent.
Biotransformation
In vitro studies with human liver microsomes and human cDNA-expressed cytochrome P450 isozymes indicate that bortezomib is primarily oxidatively metabolized via cytochrome P450 enzymes, 3A4, 2C19, and 1A2. The major metabolic pathway is deboronation to form two deboronated metabolites that subsequently undergo hydroxylation to several metabolites. Deboronated-bortezomib metabolites are inactive as 26S proteasome inhibitors.
Elimination
The mean elimination half-life (t1/2) of bortezomib upon multiple dosing ranged from 40-193 hours. Bortezomib is eliminated more rapidly following the first dose compared to subsequent doses. Mean total body clearances were 102 and 112 l/h following the first dose for doses of 1.0 mg/m² and 1.3 mg/m², respectively, and ranged from 15 to 32 l/h and 18 to 32 l/h following subsequent doses for doses of 1.0 mg/m² and 1.3 mg/m², respectively.
Special populations
Hepatic impairment: The effect of hepatic impairment on the pharmacokinetics of bortezomib was assessed in a phase I study during the first treatment cycle, including 61 patients primarily with solid tumors and varying degrees of hepatic impairment at bortezomib doses ranging from 0.5 to 1.3 mg/m².
When compared to patients with normal hepatic function, mild hepatic impairment did not alter dose-normalized bortezomib AUC. However, the dose-normalized mean AUC values were increased by approximately 60% in patients with moderate or severe hepatic impairment. A lower starting dose is recommended in patients with moderate or severe hepatic impairment, and those patients should be closely monitored (see section 4.2 Table 2).
Renal impairment: A pharmacokinetic study was conducted in patients with various degrees of renal impairment who were classified according to their creatinine clearance values (CrCL) into the following groups: Normal (CrCL ≥ 60 ml/min/1.73 m², n=12), Mild (CrCL = 40-59 ml/min/1.73 m², n = 10), Moderate (CrCL = 20-39 ml/min/1.73 m², n = 9), and Severe (CrCL < 20 ml/min/1.73 m², n = 3). A group of dialysis patients who were dosed after dialysis was also included in the study (n = 8). Patients were administered intravenous doses of 0.7 to 1.3 mg/m² of VELCADE twice weekly. Exposure of VELCADE (dose-normalized AUC and Cmax) was comparable among all the groups (see section 4.2).
Preclinical safety data
Bortezomib was positive for clastogenic activity (structural chromosomal aberrations) in the in vitro chromosomal aberration assay using Chinese hamster ovary (CHO) cells at concentrations as low as 3.125 μg/ml, which was the lowest concentration evaluated. Bortezomib was not genotoxic when tested in the in vitro mutagenicity assay (Ames assay) and in vivo micronucleus assay in mice.
Developmental toxicity studies in the rat and rabbit have shown embryo-fetal lethality at maternally toxic doses, but no direct embryo-foetal toxicity below maternally toxic doses. Fertility studies were not performed but evaluation of reproductive tissues has been performed in the general toxicity studies. In the 6-month rat study, degenerative effects in both the testes and the ovary have been observed. It is, therefore, likely that bortezomib could have a potential effect on either male or female fertility. Peri- and postnatal development studies were not conducted.
In multi-cycle general toxicity studies conducted in the rat and monkey, the principal target organs included the gastrointestinal tract, resulting in vomiting and/or diarrhoea; haematopoietic and lymphatic tissues, resulting in peripheral blood cytopenias, lymphoid tissue atrophy and haematopoietic bone marrow hypocellularity; peripheral neuropathy (observed in monkeys, mice and dogs) involving sensory nerve axons; and mild changes in the kidneys. All these target organs have shown partial to full recovery following discontinuation of treatment.
Based on animal studies, the penetration of bortezomib through the blood-brain barrier appears to be limited, if any and the relevance to humans is unknown.
Cardiovascular safety pharmacology studies in monkeys and dogs show that intravenous doses approximately two to three times the recommended clinical dose on a mg/m² basis are associated with increases in heart rate, decreases in contractility, hypotension and death. In dogs, the decreased cardiac contractility and hypotension responded to acute intervention with positive inotropic or pressor agents. Moreover, in dog studies, a slight increase in the corrected QT interval was observed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.