PAROEX Oral rinse Ref.[28053] Active ingredients: Chlorhexidine
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
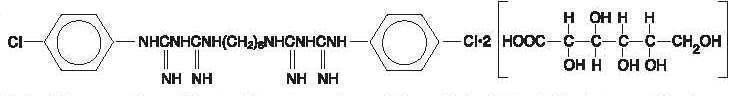
Paroex is an oral rinse containing 0.12% chlorhexidine gluconate (1,1'hexamethylene bis [5(p-chlorophenyl) biguanide] di-D-gluconate) in a base containing deionized water, propylene glycol, glycerin, polyoxyl 40 hydrogenated castor oil, mint flavor, potassium acesulfame, FD&C Red #40 and D&C Red #33. Paroex is a near-neutral solution (pH range 5-7). Chlorhexidine gluconate is a salt of chlorhexidine and gluconic acid.
Its chemical structure is:
| How Supplied |
|---|
|
Paroex is supplied as a pink liquid in the following sizes:
Manufactured for: Sunstar Americas, Inc., Chicago, IL 60630 |
Drugs
| Drug | Countries | |
|---|---|---|
| PAROEX | France, Poland, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.