TERCONAZOLE Vaginal suppository Ref.[49520] Active ingredients: Terconazole
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
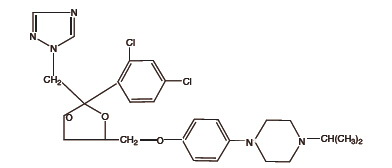
Terconazole Vaginal Suppositories are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent terconazole, cis1[p[[2(2,4-Dichlorophenyl)2(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-isopropylpiperazine, in triglycerides derived from coconut and/or palm kernel oil (a base of hydrogenated vegetable oils) and butylated hydroxyanisole.
The structural formula of terconazole is as follows:
TERCONAZOLE C26H31Cl2N5O3
Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol.
| How Supplied |
|---|
|
Terconazole Vaginal Suppositories 80 mg are available as 2.5 g, elliptically-shaped white to off-white suppositories in packages of three with a vaginal applicator. NDC 51672-1330-3 Mfd. by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 Dist. by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 Revised: August, 2014 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.