MOEXIPRIL HYDROCHLORIDE Film-coated tablet Ref.[50223] Active ingredients: Moexipril
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
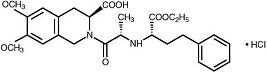
Moexipril hydrochloride USP, the hydrochloride salt of moexipril, has the empirical formula C27H34N2O7•HCl and a molecular weight of 535.04. It is chemically described as [3S-[2[R*(R*)],3R*]]2[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic acid, monohydrochloride. It is a non-sulfhydryl containing precursor of the active angiotensin-converting enzyme (ACE) inhibitor moexiprilat and its structural formula is:
Moexipril hydrochloride USP is a fine white to off-white powder. It is soluble (about 10% weight-to-volume) in distilled water at room temperature.
Moexipril hydrochloride tablets USP are supplied as scored, coated tablets containing 7.5 mg and 15 mg of moexipril hydrochloride USP for oral administration. In addition to the active ingredient, moexipril hydrochloride USP, the tablet core contains the following inactive ingredients: crospovidone, lactose monohydrate, magnesium oxide, magnesium stearate and povidone. The film coating contains: hypromellose, hydroxypropyl cellulose, titanium dioxide, polyethylene glycol 6000, magnesium stearate, ferric oxide red, ferric oxide black and ferric oxide yellow (15 mg tablet only).
| How Supplied |
|---|
|
Moexipril hydrochloride tablets USP 7.5 mg are peach, round, biconvex, film coated tablets with ‘G’ and breakline engraved on one side and ‘209’ on the other side. Bottles of 90 NDC 68462-209-90 Moexipril hydrochloride tablets USP 15 mg are brown, round, biconvex, film coated tablets with ‘G’ and breakline engraved on one side and ‘208’ on the other side. Bottles of 90 NDC 68462-208-90 Manufactured by: Glenmark Pharmaceuticals Ltd., Colvale-Bardez, Goa 403 513, India Manufactured for: Glenmark Pharmaceuticals Inc., USA, Mahwah, NJ 07430 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.