OXYCONTIN Film-coated, extended release tablet Ref.[90497] Active ingredients: Oxycodone
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
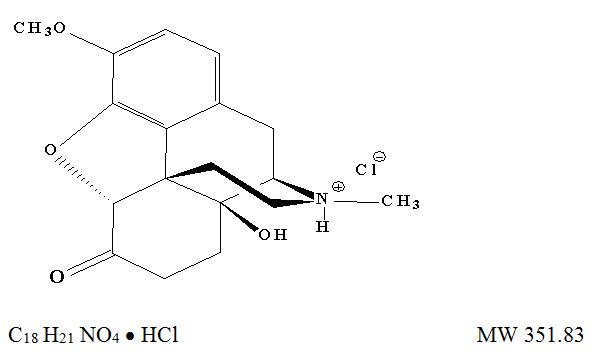
OXYCONTIN (oxycodone hydrochloride) extended-release tablets is an opioid agonist supplied in 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg, and 80 mg tablets for oral administration. The tablet strengths describe the amount of oxycodone per tablet as the hydrochloride salt. The structural formula for oxycodone hydrochloride is as follows:
The chemical name is 4, 5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride.
Oxycodone is a white, odorless crystalline powder derived from the opium alkaloid, thebaine. Oxycodone hydrochloride dissolves in water (1 g in 6 to 7 mL). It is slightly soluble in alcohol (octanol water partition coefficient 0.7).
The 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg and 80 mg tablets contain the following inactive ingredients: butylated hydroxytoluene (BHT), hypromellose, polyethylene glycol 400, polyethylene oxide, magnesium stearate, titanium dioxide.
The 10 mg tablets also contain hydroxypropyl cellulose.
The 15 mg tablets also contain black iron oxide, yellow iron oxide, and red iron oxide.
The 20 mg tablets also contain polysorbate 80 and red iron oxide.
The 30 mg tablets also contain polysorbate 80, red iron oxide, yellow iron oxide, and black iron oxide.
The 40 mg tablets also contain polysorbate 80 and yellow iron oxide.
The 60 mg tablets also contain polysorbate 80, red iron oxide and black iron oxide.
The 80 mg tablets also contain hydroxypropyl cellulose, yellow iron oxide and FD&C Blue #2/Indigo Carmine Aluminum Lake.
| Dosage Forms and Strengths |
|---|
|
Extended-release tablets: 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg, and 80 mg.
|
| How Supplied |
|---|
|
OXYCONTIN (oxycodone hydrochloride) extended-release tablets 10 mg are film-coated, round, white-colored, bi-convex tablets debossed with OP on one side and 10 on the other and are supplied as child-resistant closure, opaque plastic bottles of 100 (NDC 59011-410-10) and unit dose packaging with 10 individually numbered tablets per card; two cards per glue end carton (NDC 59011-410-20). OXYCONTIN (oxycodone hydrochloride) extended-release tablets 15 mg are film-coated, round, gray-colored, bi-convex tablets debossed with OP on one side and 15 on the other and are supplied as child-resistant closure, opaque plastic bottles of 100 (NDC 59011-415-10) and unit dose packaging with 10 individually numbered tablets per card; two cards per glue end carton (NDC 59011-415-20). OXYCONTIN (oxycodone hydrochloride) extended-release tablets 20 mg are film-coated, round, pink-colored, bi-convex tablets debossed with OP on one side and 20 on the other and are supplied as child-resistant closure, opaque plastic bottles of 100 (NDC 59011-420-10) and unit dose packaging with 10 individually numbered tablets per card; two cards per glue end carton (NDC 59011-420-20). OXYCONTIN (oxycodone hydrochloride) extended-release tablets 30 mg are film-coated, round, brown-colored, bi-convex tablets debossed with OP on one side and 30 on the other and are supplied as child-resistant closure, opaque plastic bottles of 100 (NDC 59011-430-10) and unit dose packaging with 10 individually numbered tablets per card; two cards per glue end carton (NDC 59011-430-20). OXYCONTIN (oxycodone hydrochloride) extended-release tablets 40 mg are film-coated, round, yellow-colored, bi-convex tablets debossed with OP on one side and 40 on the other and are supplied as child-resistant closure, opaque plastic bottles of 100 (NDC 59011-440-10) and unit dose packaging with 10 individually numbered tablets per card; two cards per glue end carton (NDC 59011-440-20). OXYCONTIN (oxycodone hydrochloride) extended-release tablets 60 mg are film-coated, round, red-colored, bi-convex tablets debossed with OP on one side and 60 on the other and are supplied as child-resistant closure, opaque plastic bottles of 100 (NDC 59011-460-10) and unit dose packaging with 10 individually numbered tablets per card; two cards per glue end carton (NDC 59011-460-20). OXYCONTIN (oxycodone hydrochloride) extended-release tablets 80 mg are film-coated, round, green-colored, bi-convex tablets debossed with OP on one side and 80 on the other and are supplied as child-resistant closure, opaque plastic bottles of 100 (NDC 59011-480-10) and unit dose packaging with 10 individually numbered tablets per card; two cards per glue end carton (NDC 59011-480-20). Purdue Pharma L.P., Stamford, CT 06901-3431 |
Drugs
| Drug | Countries | |
|---|---|---|
| OXYCONTIN | Austria, Australia, Brazil, Cyprus, Ecuador, Estonia, Spain, Finland, France, Hong Kong, Croatia, Ireland, Japan, Mexico, Netherlands, New Zealand, Poland, Singapore, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.