BEOVU Solution for injection Ref.[9918] Active ingredients: Brolucizumab
Source: FDA, National Drug Code (US) Revision Year: 2020
1. Indications and Usage
BEOVU is indicated for the treatment of Neovascular (Wet) Age-related Macular Degeneration (AMD).
2. Dosage and Administration
2.1 General Dosing Information
For ophthalmic intravitreal injection. BEOVU must be administered by a qualified physician.
2.2 Neovascular (Wet) Age-Related Macular Degeneration (AMD)
The recommended dose for BEOVU is 6 mg (0.05 mL of 120 mg/mL solution) administered by intravitreal injection monthly (approximately every 25-31 days) for the first three doses, followed by 6 mg (0.05 mL) by intravitreal injection once every 8-12 weeks.
2.3 Preparation for Administration
 | Store BEOVU in the refrigerator between 2°C to 8°C (36°F to 46°F); do not freeze. Keep the vial in the outer carton to protect from light. |
 | Prior to use, the unopened glass vial of BEOVU may be kept at room temperature, 20°C to 25°C (68°F to 77°F) for up to 24 hours. After opening the glass vial, proceed under aseptic conditions. |
 | BEOVU is a clear to slightly opalescent and colorless to slightly brownish-yellow solution. |
 | BEOVU should be inspected visually upon removal from the refrigerator and prior to administration. If particulates, cloudiness, or discoloration are visible, the glass vial must not be used. The BEOVU kit includes the sterile glass vial and filter needle which are for single use only. Do not use if the packaging, vial and/or filter needle are damaged or expired [see How Supplied/Storage and Handling (16)]. |
Use aseptic technique for preparation of the intravitreal injection.
| STEP 1: Gather the supplies needed. • One BEOVU vial (included) • One sterile 5-micron blunt filter needle (18-gauge x 1½ inch, 1.2 mm x 40 mm) (included) • One sterile 30-gauge x ½ inch injection needle (not included) • One sterile 1 mL syringe with a 0.05 mL dose mark (not included) • Alcohol swab (not included) | |
| STEP 2 Allow vial to come to room temperature and inspect the solution. If particulates, cloudiness, or discoloration are visible, discard the vial and obtain a new vial. | |
| STEP 3 Remove the vial cap and clean the vial septum (e.g., with alcohol swab) (see Figure 1). | 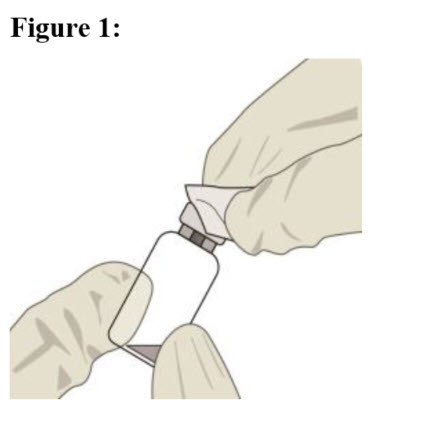 |
| STEP 4 Assemble the 5-micron filter needle (18-gauge x 1½ inch) onto a 1-mL syringe using aseptic technique. | |
| STEP 5 Push the filter needle into the center of the vial septum until the needle touches the bottom of the vial. | |
| STEP 6 To withdraw the liquid, hold the vial slightly inclined and slowly withdraw all the liquid from the vial and filter needle (see Figure 2). Ensure that the plunger rod is drawn sufficiently back when emptying the vial in order to completely empty the filter needle. | 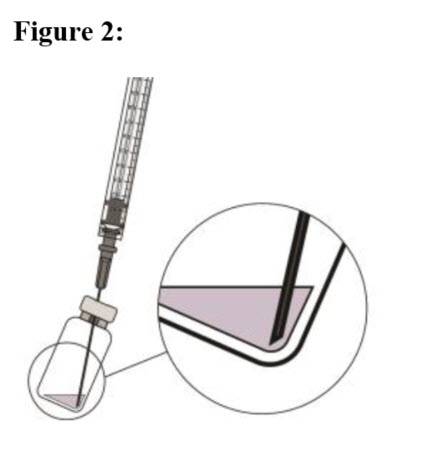 |
| STEP 7 Disconnect the filter needle from the syringe in an aseptic manner and dispose of it. The filter needle is not to be used for the intravitreal injection. | |
| STEP 8 Aseptically and firmly assemble a 30-gauge x ½ inch injection needle onto the syringe. | |
| STEP 9 Check for air bubbles by holding the syringe with the needle pointing up. If there are any air bubbles, gently tap the syringe with your finger until the bubbles rise to the top (see Figure 3). | 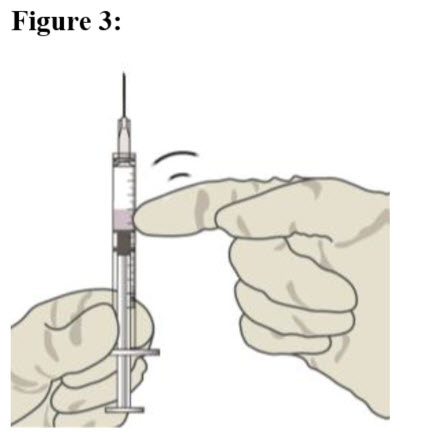 |
| STEP 10 Carefully expel the air from the syringe and adjust the dose to the 0.05 mL mark (see Figure 4). The syringe is ready for the injection. | 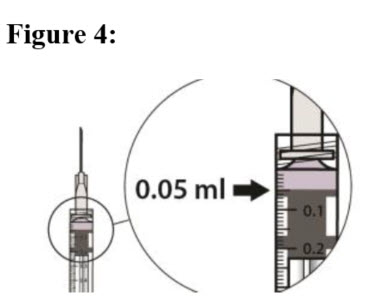 |
2.4 Injection Procedure
Ensure that the injection is given immediately after preparation of the dose.
The intravitreal injection procedure must be carried out under aseptic conditions, which includes the use of surgical hand disinfection, sterile gloves, a sterile drape and a sterile eyelid speculum (or equivalent), and the availability of sterile paracentesis equipment (if required). Adequate anesthesia and a broad-spectrum topical microbicide to disinfect the periocular skin, eyelid, and ocular surface should be administered prior to the injection.
Inject slowly until the rubber stopper reaches the end of the syringe to deliver the volume of 0.05 mL. Confirm delivery of the full dose by checking that the rubber stopper has reached the end of the syringe barrel.
Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure (IOP). Appropriate monitoring may consist of a check for perfusion of the optic nerve head or tonometry. If required, a sterile paracentesis needle should be available.
Following intravitreal injection, patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment (e.g., eye pain, redness of the eye, photophobia, blurring of vision) without delay [see Patient Counseling Information (17)].
Each vial should only be used for the treatment of a single eye. If the contralateral eye requires treatment, a new vial should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, filter, and injection needles should be changed before BEOVU is administered to the other eye.
Any unused medicinal product or waste material should be disposed of in accordance with local regulations.
16.1. Storage and Handling
Refrigerate BEOVU between 2°C to 8°C (36°F to 46°F). Do not freeze. Store the vial in the outer carton to protect from light.
Prior to use, the unopened glass vial of BEOVU may be kept at room temperature, 20°C to 25°C (68°F to 77°F) for up to 24 hours.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.