GIVLAARI Solution for injection Ref.[9931] Active ingredients: Givosiran
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
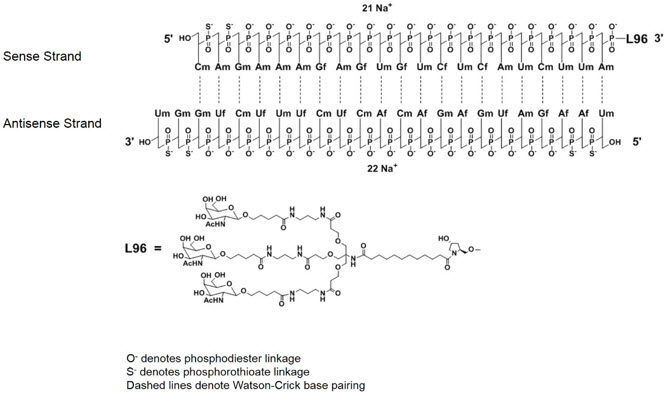
GIVLAARI is an aminolevulinate synthase 1-directed small interfering RNA (siRNA), covalently linked to a ligand containing three N-acetylgalactosamine (GalNAc) residues to enable delivery of the siRNA to hepatocytes.
The structural formulas of the givosiran drug substance in its sodium form, and the ligand (L96), are presented below.
Abbreviations: Af = adenine 2'-F ribonucleoside; Cf = cytosine 2'-F ribonucleoside; Uf = uracil 2'-F ribonucleoside; Am = adenine 2'-OMe ribonucleoside; Cm = cytosine 2'-OMe ribonucleoside; Gf = guanine 2'-F ribonucleoside; Gm = guanine 2'-OMe ribonucleoside; Um = uracil 2'-OMe ribonucleoside; L96 = triantennary GalNAc (N-acetylgalactosamine)
GIVLAARI is supplied as a sterile, preservative-free, 1-mL colorless-to-yellow solution for subcutaneous injection containing 189 mg givosiran in a single-dose, 2-mL Type 1 glass vial with a TEFLON-coated stopper and a flip-off aluminum seal. GIVLAARI is available in cartons containing one single-dose vial each. Water for injection is the only excipient used in the manufacture of GIVLAARI.
The molecular formula of givosiran sodium is C524H651F16N173Na43O316P43S6 with a molecular weight of 17,245.56 Da.
The molecular formula of givosiran (free acid) is C524H694F16N173O316P43S6 with a molecular weight of 16,300.34 Da.
| Dosage Forms and Strengths |
|---|
|
Injection: 189 mg/mL clear, colorless-to-yellow solution in a single-dose vial. |
| How Supplied |
|---|
|
GIVLAARI (givosiran) is a clear, colorless-to-yellow ready-to-use solution available in single-dose vials of 189 mg/mL in cartons containing one vial (NDC 71336-1001-1). |
Drugs
| Drug | Countries | |
|---|---|---|
| GIVLAARI | Austria, Brazil, Canada, Cyprus, Estonia, Spain, France, Croatia, Ireland, Israel, Italy, Japan, Lithuania, Poland, Romania, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.