NUBEQA Film-coated tablet Ref.[9932] Active ingredients: Darolutamide
Source: FDA, National Drug Code (US) Revision Year: 2019
12.1. Mechanism of Action
Darolutamide is an androgen receptor (AR) inhibitor. Darolutamide competitively inhibits androgen binding, AR nuclear translocation, and AR-mediated transcription. A major metabolite, keto-darolutamide, exhibited similar in vitro activity to darolutamide. In addition, darolutamide functioned as a progesterone receptor (PR) antagonist in vitro (approximately 1% activity compared to AR). Darolutamide decreased prostate cancer cell proliferation in vitro and tumor volume in mouse xenograft models of prostate cancer.
12.2. Pharmacodynamics
Darolutamide exposure at 600 mg twice daily results in PSA mean reduction of more than 90% from baseline.
Cardiac Electrophysiology
The effect of darolutamide (600 mg twice daily) on the QTc interval was evaluated in a subgroup of 500 patients in the ARAMIS study. No large mean increase in QTc (i.e., >20 ms) was detected.
12.3. Pharmacokinetics
Following administration of 600 mg twice daily, darolutamide mean (%CV) steady-state peak plasma concentration (Cmax) is 4.79 mg/L (30.9%) and area under the plasma concentration-time curve from time 0 to 12 hours (AUC12h) is 52.82 h•µg/mL (33.9%). Steady-state is reached 2–5 days after repeated dosing with food, with an approximate 2-fold accumulation.
The exposure (Cmax and AUC12) of the darolutamide and the active metabolite keto‑darolutamide increase in a nearly dose-proportional manner in the dose range of 100 to 700 mg (0.17 to 1.17 times the approved recommended dosage). No further increase in darolutamide exposure was observed at 900 mg twice daily (1.5 times the approved recommended dosage).
Absorption
Darolutamide Cmax is reached approximately 4 hours after administration of a single 600 mg oral dose.
The absolute bioavailability is approximately 30% following oral administration of a NUBEQA tablet containing 300 mg darolutamide under fasted conditions.
Food Effect
Bioavailability of darolutamide increased by 2.0 to 2.5‑fold when administered with food. A similar increase of exposure was observed for the active metabolite keto‑darolutamide.
Distribution
The apparent volume of distribution of darolutamide after intravenous administration is 119 L.
Protein binding is 92% for darolutamide and 99.8% for the active metabolite, keto‑darolutamide. Serum albumin is the main binding protein for darolutamide and keto-darolutamide.
Elimination
The effective half-life of darolutamide and keto‑darolutamide is approximately 20 hours in patients. The clearance (%CV) of darolutamide following intravenous administration is 116 mL/min (39.7%).
Metabolism
Darolutamide is primarily metabolized by CYP3A4, as well as by UGT1A9 and UGT1A1. Keto‑darolutamide total exposure in plasma is 1.7‑fold higher compared to darolutamide.
Excretion
After a single radiolabeled dose as an oral solution, a total of 63.4% of darolutamide‑related material is excreted in the urine (approximately 7% unchanged) and 32.4% (approximately 30% unchanged) in the feces. More than 95% of the dose was recovered within 7 days after administration.
Specific Populations
In nmCRPC patients, no clinically significant differences in the pharmacokinetics of darolutamide were observed based on age (48-95 years), race (White, Japanese, non-Japanese Asian, Black or African American), mild to moderate renal impairment (eGFR 30–89 mL/min/1.73m²), or mild hepatic impairment.
In non-cancer subjects with severe renal impairment (eGFR 15–29 mL/min/1.73 m²) not receiving dialysis or with moderate hepatic impairment (Child-Pugh Class B), NUBEQA exposure increased by about 2.5- and 1.9-fold, respectively, compared to healthy subjects.
The effect of end-stage renal disease (eGFR <15 mL/min/1.73 m²) or severe hepatic impairment (Child-Pugh C) on darolutamide pharmacokinetics has not been studied.
Drug Interaction Studies
Clinical Studies
Combined P-gp and Strong CYP3A4 Inducers: Concomitant use of rifampicin (a combined P-gp and strong CYP3A4 inducer) decreased mean darolutamide AUC0-72 by 72% and Cmax by 52%. The decrease of darolutamide exposure by moderate CYP3A4 inducers is expected to be in the range of 36%-58%.
Combined P-gp and Strong CYP3A4 Inhibitors
Itraconazole (a strong combined CYP3A4 and P-gp inhibitor) increased mean darolutamide AUC0-72 by 1.7- and Cmax by 1.4-fold.
CYP3A4 substrates
Concomitant use of darolutamide decreased the mean AUC and Cmax of midazolam (CYP3A4 substrate) by 29% and 32%, respectively. No clinically significant differences in the pharmacokinetics of midazolam were observed when used concomitantly with darolutamide.
BCRP Substrates
Concomitant use of darolutamide increased the mean AUC and Cmax of rosuvastatin (BCRP substrate) by approximately 5-fold.
P-gp Substrates
No clinically significant differences in the pharmacokinetics of dabigatran (P-gp substrate) were observed when used concomitantly with darolutamide.
In Vitro Studies
In vitro, darolutamide inhibits OATP1B1 and OATP1B3. Darolutamide did not inhibit the major CYP enzymes (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4) or transporters (MRP2, BSEP, OATs, OCTs, MATEs, OATP2B1, and NTCP) at clinically relevant concentrations.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of darolutamide have not been conducted.
Darolutamide was clastogenic in an in vitro chromosome aberration assay in human peripheral blood lymphocytes. Darolutamide did not induce mutations in the bacterial reverse mutation (Ames) assay and was not genotoxic in the in vivo combined bone marrow micronucleus assay and the Comet assay in the liver and duodenum of the rat.
Fertility studies in animals have not been conducted with darolutamide. In repeat-dose toxicity studies in male rats (up to 26 weeks) and dogs (up to 39 weeks), tubular dilatation of testes, hypospermia, and atrophy of seminal vesicles, testes, prostate gland and epididymides were observed at doses ≥100 mg/kg/day in rats (0.6 times the human exposure based on AUC) and ≥50 mg/kg/day in dogs (approximately 1 times the human exposure based on AUC).
14. Clinical Studies
ARAMIS (NCT02200614) was a multicenter, double-blind, placebo-controlled clinical trial in 1509 patients with non-metastatic castration resistant prostate cancer with a prostate-specific antigen doubling time (PSADT) of ≤10 months. Randomization was stratified by PSADT and use of bone-targeted therapy at study entry. Patients with pelvic lymph nodes less than 2 cm in short axis below the aortic bifurcation were allowed to enter the study. Patients with a history of seizures were not excluded. Absence or presence of metastasis was assessed by blinded independent central review (BICR). PSA results were not blinded and were not used for treatment discontinuation.
Patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n=955) or matching placebo (n=554). Treatment continued until radiographic disease progression as assessed by CT, MRI, 99mTc bone scan by BICR, unacceptable toxicity or withdrawal. All patients received a gonadotropin-releasing hormone (GnRH) analog concurrently or had a bilateral orchiectomy.
The following patient demographics and disease characteristics were balanced between treatment arms. The median age was 74 years (range 48–95) and 9% of patients were 85 years of age or older. The racial distribution was 79% White, 13% Asian, and 3% Black. A majority of patients (73%) had a Gleason score of 7 or higher at diagnosis. The median PSADT was 4.5 months. Forty-two percent of patients in both treatment arms had prior surgery or radiotherapy to the prostate. Eleven percent of patients had enlarged pelvic lymph nodes less than 2 cm at study entry. Six percent of patients were retrospectively identified by BICR as having metastases at baseline. Seventy-three percent of patients received prior treatment with an anti-androgen (bicalutamide or flutamide). All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 at study entry. There were 12 patients enrolled on the NUBEQA arm with a history of seizure. At baseline, 47% of patients reported no pain on the Brief Pain Inventory-Short Form (a 7-day diary average of the daily worst pain item).
The major efficacy endpoint was metastasis free survival (MFS), defined as the time from randomization to the time of first evidence of BICR-confirmed distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first. Distant metastasis was defined as new bone or soft tissue lesions or enlarged lymph nodes above the aortic bifurcation. Overall survival (OS) and time to pain progression were additional efficacy endpoints.
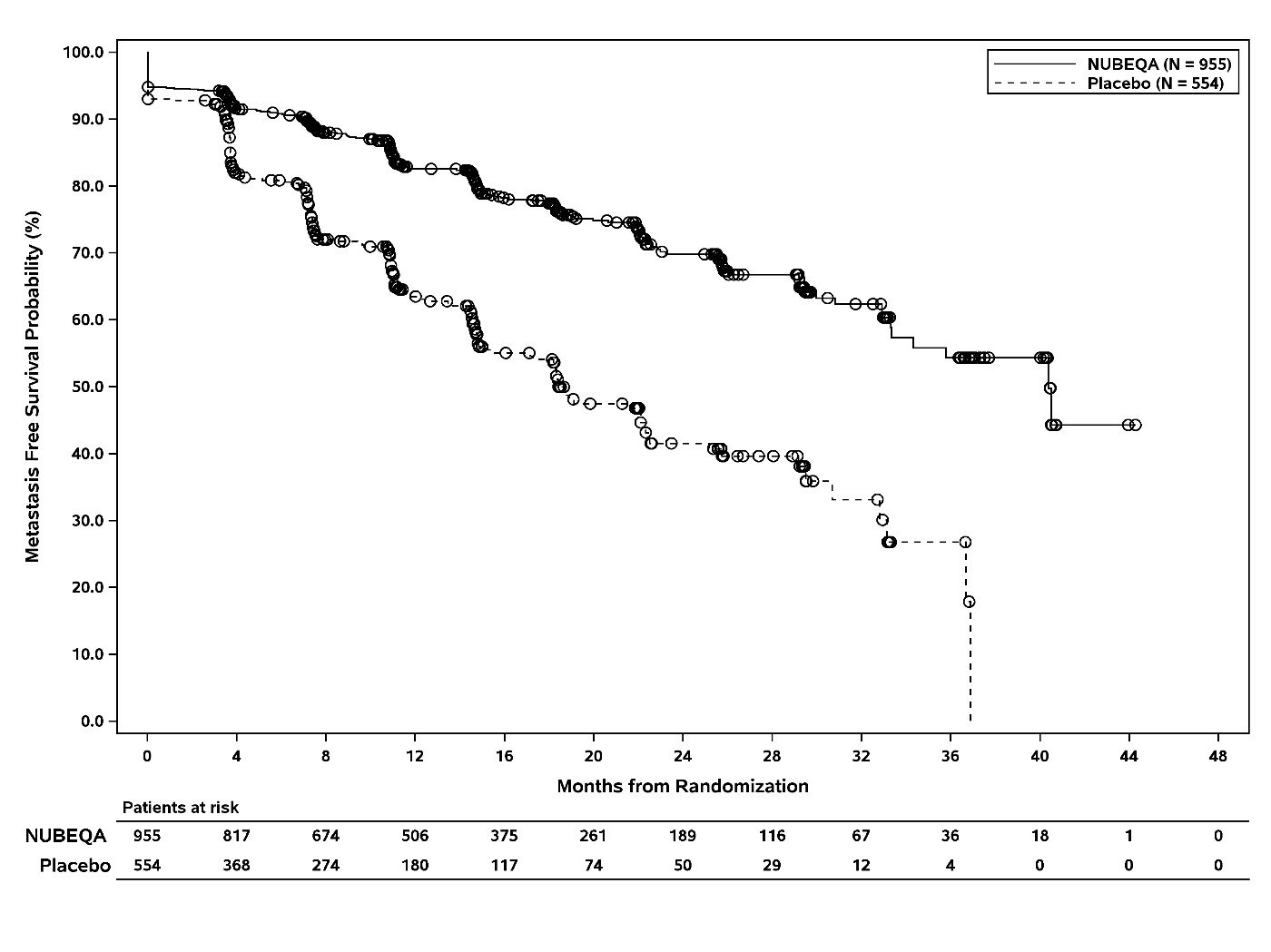
The efficacy results for MFS from ARAMIS are summarized in Table 3 and Figure 1. Treatment with NUBEQA resulted in a statistically significant improvement in MFS compared to placebo. MFS results were consistent across patient subgroups for PSADT (≤6 months or >6 months) or prior use of bone-targeting agents (yes or no). OS data were not mature at the time of final MFS analysis (57% of the required number of events). Locoregional-only progression occurred in 6% of patients overall.
Table 3. Efficacy Results from the ARAMIS Study:
| NUBEQA (N=955) | Placebo (N=554) | |
|---|---|---|
| Metastasis-free survival | ||
| Number of Events (%) | 221 (23) | 216 (39) |
| Median, months (95% CI)* | 40.4 (34.3, NR†) | 18.4 (15.5, 22.3) |
| Hazard Ratio (95% CI)‡ | 0.41 (0.34, 0.50) | |
| P-value§ | <0.0001 | |
* Based on Kaplan-Meier estimates
† NR: not reached
‡ Hazard ratio is based on a Cox regression model (with treatment as the only covariate) stratified by PSADT (≤6 months vs. >6 months) and use of osteoclast-targeted therapy (yes vs. no). Hazard ratio <1 favors NUBEQA
§ P-value is based on a stratified log-rank test by PSADT (≤6 months vs. >6 months) and use of osteoclasttargeted therapy (yes vs. no)
Figure 1. Kaplan Meier Curve Metastasis Free Survival:
The MFS result was supported by a delay in time to pain progression, defined as at least a 2-point worsening from baseline of the pain score on Brief Pain Inventory-Short Form or initiation of opioids, in patients treated with NUBEQA as compared to placebo. Pain progression was reported in 28% of all patients on study.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.