ALUNBRIG Film-coated tablet Ref.[9973] Active ingredients: Brigatinib
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Brigatinib is a tyrosine kinase inhibitor with in vitro activity at clinically achievable concentrations against multiple kinases including ALK, ROS1, insulin-like growth factor-1 receptor (IGF-1R), and FLT-3 as well as EGFR deletion and point mutations. Brigatinib inhibited autophosphorylation of ALK and ALK-mediated phosphorylation of the downstream signaling proteins STAT3, AKT, ERK1/2, and S6 in in vitro and in vivo assays. Brigatinib also inhibited the in vitro proliferation of cell lines expressing EML4-ALK and NPM-ALK fusion proteins and demonstrated dose-dependent inhibition of EML4-ALK-positive NSCLC xenograft growth in mice.
At clinically achievable concentrations (≤500 nM), brigatinib inhibited the in vitro viability of cells expressing EML4-ALK and 17 mutant forms associated with resistance to ALK inhibitors including crizotinib, as well as EGFR-Del (E746-A750), ROS1-L2026M, FLT3-F691L, and FLT3-D835Y. Brigatinib exhibited in vivo antitumor activity against 4 mutant forms of EML4-ALK, including G1202R and L1196M mutants identified in NSCLC tumors in patients who have progressed on crizotinib. Brigatinib also reduced tumor burden and prolonged survival in mice implanted intracranially with an ALK-driven tumor cell line.
12.2. Pharmacodynamics
Brigatinib exposure-response relationships and the time course of the pharmacodynamic response are unknown.
Cardiac Electrophysiology
The QT interval prolongation potential of ALUNBRIG was assessed in 123 patients following once daily ALUNBRIG doses of 30 mg (0.16 times the recommended dose of 180 mg) to 240 mg (1.3 times the recommended dose of 180 mg). ALUNBRIG did not prolong the QT interval to a clinically relevant extent.
12.3. Pharmacokinetics
The geometric mean (CV%) steady-state maximum concentration (Cmax) of brigatinib at ALUNBRIG doses of 90 mg and 180 mg once daily was 552 (49%) ng/mL and 1452 (60%) ng/mL, respectively, and the corresponding area under the concentration-time curve (AUC0-Tau) was 8165 (45%) ng∙h/mL and 20276 (62%) ng∙h/mL. After a single dose and multiple dosing of ALUNBRIG, systemic exposure of brigatinib was dose proportional over the dose range of 60 mg (0.3 times the recommended dose of 180 mg) to 240 mg (1.3 times the recommended dose of 180 mg) once daily. The mean accumulation ratio after repeat dosing was 1.9 to 2.4.
Absorption
Following administration of single oral doses of ALUNBRIG of 30 mg to 240 mg, the median time to peak concentration (Tmax) ranged from 1 to 4 hours.
Effect of Food
Brigatinib Cmax was reduced by 13% with no effect on AUC in healthy subjects administered ALUNBRIG after a high fat meal (approximately 920 calories, 58 grams carbohydrate, 59 grams fat and 40 grams protein) compared to the Cmax and AUC after overnight fasting.
Distribution
Brigatinib is 91% bound to human plasma proteins and the binding is not concentration-dependent. The blood-to-plasma concentration ratio is 0.69. Following oral administration of ALUNBRIG 180 mg once daily, the mean apparent volume of distribution (Vz/F) of brigatinib at steady-state was 307 L.
Elimination
Following oral administration of ALUNBRIG 180 mg once daily, the mean apparent oral clearance (CL/F) of brigatinib at steady-state is 8.9 L/h and the mean plasma elimination half-life is 25 hours.
Metabolism
Brigatinib is primarily metabolized by CYP2C8 and CYP3A4 in vitro. Following oral administration of a single 180 mg dose of radiolabeled brigatinib to healthy subjects, N-demethylation and cysteine conjugation were the two major metabolic pathways. Unchanged brigatinib (92%) was the major circulating radioactive component.
Excretion
Following oral administration of a single 180 mg dose of radiolabeled brigatinib to healthy subjects, 65% of the administered dose was recovered in feces and 25% of the administered dose was recovered in urine. Unchanged brigatinib represented 41% and 86% of the total radioactivity in feces and urine, respectively.
Specific Populations
Age, race, sex, body weight, and albumin concentration have no clinically meaningful effect on the pharmacokinetics of brigatinib.
Patients with Hepatic Impairment
Following a single dose of ALUNBRIG 90 mg, unbound brigatinib systemic exposure (AUC0-INF) was 37% higher in subjects with severe hepatic impairment (Child-Pugh C) compared to subjects with normal hepatic function. Unbound brigatinib systemic exposure (AUC0-INF) was similar between subjects with mild (Child-Pugh A) to moderate (Child-Pugh B) hepatic impairment and subjects with normal hepatic function [see Dosage and Administration (2.6)].
Patients with Renal Impairment
Following a single dose of ALUNBRIG 90 mg, unbound brigatinib systemic exposure (AUC0-INF) was 86% higher in subjects with severe renal impairment [creatinine clearance (CLcr) 15 to 29 mL/min] compared to subjects with normal renal function.
Based on a population pharmacokinetic analysis, brigatinib exposures were similar among 125 subjects with mild renal impairment (CLcr 60 to 89 mL/min), 34 subjects with moderate renal impairment (CLcr 30 to 59 mL/min) and 270 subjects with normal renal function (CLcr ≥90 mL/min) [see Dosage and Administration (2.7)].
Drug Interaction Studies
Clinical Studies
Effect of Strong and Moderate CYP3A Inhibitors on Brigatinib:
Coadministration of 200 mg twice daily doses of itraconazole (a strong CYP3A inhibitor) with a single 90 mg dose of ALUNBRIG increased brigatinib Cmax by 21% and AUC0-INF by 101%, relative to a 90 mg dose of ALUNBRIG administered alone [see Dosage and Administration (2.4), Drug Interactions (7.1)]. A moderate CYP3A inhibitor is predicted to increase the AUC of brigatinib by approximately 40%.
Effect of Strong CYP2C8 Inhibitors on Brigatinib:
Coadministration of 600 mg twice daily doses of gemfibrozil (a strong CYP2C8 inhibitor) with a single 90 mg dose of ALUNBRIG decreased brigatinib Cmax by 41% and AUC0-INF by 12%, relative to a 90 mg dose of ALUNBRIG administered alone. The effect of gemfibrozil on the pharmacokinetics of brigatinib is not clinically meaningful and the underlying mechanism for the decreased exposure of brigatinib is unknown.
Effect of Strong and Moderate CYP3A Inducers on Brigatinib:
Coadministration of 600 mg daily doses of rifampin (a strong CYP3A inducer) with a single 180 mg dose of ALUNBRIG decreased brigatinib Cmax by 60% and AUC0-INF by 80%, relative to a 180 mg dose of ALUNBRIG administered alone [see Dosage and Administration (2.5), Drug Interactions (7.1)]. A moderate CYP3A inducer is predicted to decrease the AUC of brigatinib by approximately 50%.
In Vitro Studies
Effect of Brigatinib on CYP Enzymes:
Brigatinib, at clinically relevant plasma concentrations, induced CYP3A via activation of the pregnane X receptor (PXR). Brigatinib may also induce CYP2C enzymes via the same mechanism at clinically relevant concentrations.
Brigatinib did not inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4/5 at clinically relevant concentrations.
Effect of P-glycoprotein and BCRP Inhibitors on Brigatinib:
Brigatinib is a substrate of the efflux transporters P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). Given that brigatinib exhibits high solubility and high permeability in vitro, P-gp and BCRP inhibitors are unlikely to increase plasma concentrations of brigatinib.
Effect of Other Transporters on Brigatinib:
Brigatinib is not a substrate of organic anion transporting polypeptide (OATP1B1, OATP1B3), organic anion transporter (OAT1, OAT3), organic cation transporter (OCT1, OCT2), multidrug and toxin extrusion protein (MATE1, MATE2K), or bile salt export pump (BSEP).
Effect of Brigatinib on Transporters:
Brigatinib is an inhibitor of P-gp, BCRP, OCT1, MATE1, and MATE2K in vitro. Therefore, brigatinib may have the potential to increase concentrations of coadministered substrates of these transporters. Brigatinib at clinically relevant concentrations did not inhibit OATP1B1, OATP1B3, OAT1, OAT3, OCT2 or BSEP.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with brigatinib.
Treatment with brigatinib resulted in chromosomal damage in an in vivo mammalian erythrocyte micronucleus in the rat, but was not mutagenic in the Ames or in vitro mammalian chromosome aberration tests.
Dedicated animal fertility studies were not conducted with brigatinib. Testicular toxicity was observed in repeat-dose animal studies at doses resulting in exposure as low as 0.2 times the exposure in patients at the 180 mg dose. In rats, findings included lower weight of testes, seminal vesicles and prostate gland, and testicular tubular degeneration; these effects were not reversible during the 2 month recovery period. In monkeys, findings included reduced size of testes along with microscopic evidence of hypospermatogenesis; these effects were reversible during the recovery period.
14. Clinical Studies
TKI-naïve Advanced ALK-positive NSCLC (ALTA 1L Study)
The efficacy of ALUNBRIG was demonstrated in a randomized (1:1), open-label, multicenter trial (ALTA 1L, NCT02737501) in adult patients with advanced ALK-positive NSCLC who had not previously received an ALK-targeted therapy. The study required patients to have an ALK rearrangement based on a local standard of care testing. Eligible patients were allowed to have up to 1 prior regimen of chemotherapy in the locally advanced or metastatic setting and were required to have an ECOG Performance Status of 0-2. Neurologically stable patients with treated or untreated central nervous system (CNS) metastases, including leptomeningeal metastases, were eligible. Patients with a history of interstitial lung disease, drug-related pneumonitis, or radiation pneumonitis were excluded. The major efficacy outcome measure was progression-free survival (PFS) as evaluated by a Blinded Independent Review Committee (BIRC) according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1). Additional efficacy outcome measures as evaluated by the BIRC include confirmed overall response rate (ORR), duration of response (DOR), intracranial ORR, and intracranial DOR.
A total of 275 patients were randomized to receive ALUNBRIG 180 mg orally once daily with a 7-day lead-in at 90 mg once daily (n=137) or crizotinib 250 mg orally twice daily (n=138). Of the 275 enrolled patients, 239 had positive results using the companion diagnostic test, the Vysis ALK Break Apart FISH Probe Kit; central results were negative for 20 patients and unavailable for 16 patients.
Randomization was stratified by CNS metastases (present vs absent) and prior chemotherapy use for locally advanced or metastatic disease (yes, no).
Baseline demographic characteristics of the overall study population were: median age 59 years (range: 27-89, 32% 65 and over), 59% White and 39% Asian, 55% female, 39% ECOG PS 0 and 56% ECOG PS 1, and 58% never smokers. The disease characteristics of the overall study population were: 93% with Stage IV disease; 27% received chemotherapy in the locally advanced or metastatic setting; 14% had received CNS radiation; 31% had bone metastases; and 20% had liver metastases. CNS metastases were present in 35% (n=96) of patients; 41 of those patients had measurable CNS lesions.
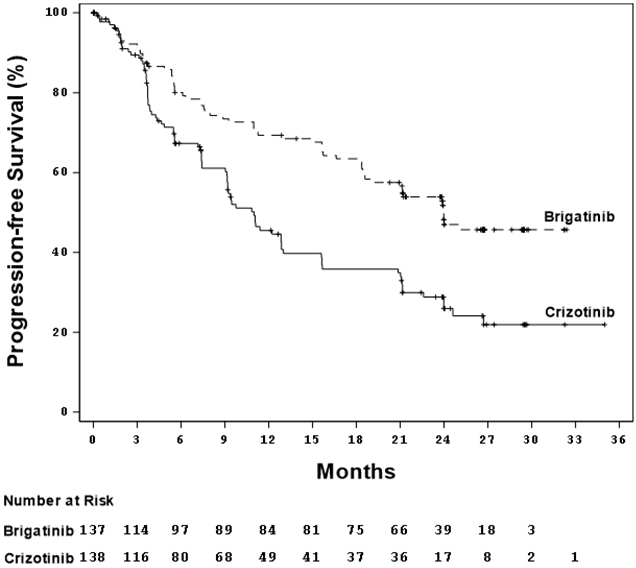
ALTA 1L demonstrated a statistically significant improvement in PFS by BIRC. Efficacy results from this study are described in Table 7 and Figure 1.
At the data cutoff point overall survival data was not mature.
Table 7. Efficacy Results by BIRC Assessment in ALTA 1L:
| Efficacy Parameters | ALUNBRIG N=137 | Crizotinib N=138 |
|---|---|---|
| Progression-free survival | ||
| Number of Events, n (%) | 63 (46%) | 87 (63%) |
| Median (months) (95% CI) | 24.0 (18.5, NE) | 11.0 (9.2, 12.9) |
| Hazard ratio (95% CI) | 0.49 (0.35, 0.68) | |
| p-value* | <.0001 | |
| Tumor Responses | ||
| Confirmed Overall Response Rate, % (95% CI) | 74% (66, 81) | 62% (53, 70) |
| p-value* | 0.0342 | |
| Complete Response, % (95% CI) | 15% (9, 22) | 9% (5, 15) |
| Partial Response, % (95% CI) | 59% (50, 67) | 53% (44, 61) |
| Duration of Response | ||
| Number of Confirmed responders | n=101 | n=85 |
| Median (months) (95% CI) | NR (19.4, NE) | 13.8 (9.3, 20.8) |
| Response duration ≥24 months | 51% | 30% |
CI = Confidence Interval, NR=Not Reached, NE=Not Estimable
* Stratified by presence of iCNS metastases at baseline and prior chemotherapy for locally advanced or metastatic disease for log-rank test and Cochran MantelHaenszel test, respectively
Figure 1. Kaplan-Meier Plot of Progression-Free Survival by BIRC in ALTA 1L:
BIRC assessment of confirmed intracranial ORR and intracranial DOR according to RECIST v1.1 in the subgroup of 41 patients with measurable CNS metastases (≥10 mm in longest diameter) at baseline are summarized in Table 8. Duration of intracranial response was measured from date of first intracranial response until intracranial disease progression (new lesions, intracranial target lesion diameter growth ≥20% from nadir, or unequivocal progression of intracranial nontarget lesions) or death.
Table 8. Intracranial Overall Response in Patients with Measurable CNS Metastases in ALTA 1L:
| Efficacy Parameter | ALUNBRIG (N=18) | Crizotinib (N=23) |
|---|---|---|
| Confirmed Intracranial Overall Response Rate, (95% CI) | 78% (52, 94) | 26% (10, 48) |
| Complete Response, % (95% CI) | 28% (10, 53) | 0 (0, 15) |
| Partial Response, % (95% CI) | 50% (26, 74) | 26% (10, 48) |
| Duration of Intracranial Response | ||
| Number of Confirmed Responders | n=14 | n=6 |
| Intracranial Response Duration ≥24 months | 64% | NE |
CI = Confidence Interval; NE = Not Estimable
ALK-positive Advanced or Metastatic NSCLC Previously Treated with Crizotinib
The efficacy of ALUNBRIG was demonstrated in a two-arm, open-label, multicenter trial (ALTA, NCT02094573) in adult patients with locally advanced or metastatic ALK-positive non-small cell lung cancer (NSCLC) who had progressed on crizotinib. The study required patients to have a documented ALK rearrangement based on an FDA-approved test or a different test with adequate archival tissue to confirm ALK arrangement by the Vysis ALK Break-Apart fluorescence in situ hybridization (FISH) Probe Kit test. Key eligibility criteria included an ECOG Performance Status of 0-2 and progression on crizotinib. Neurologically stable patients with central nervous system (CNS) metastases were permitted to enroll. Patients with a history of interstitial lung disease or drug-related pneumonitis or who had received crizotinib within 3 days of the first dose of brigatinib were excluded. The major efficacy outcome measure was confirmed overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1) as evaluated by an Independent Review Committee (IRC). Additional efficacy outcome measures included Investigator-assessed ORR, duration of response (DOR), intracranial ORR, and intracranial DOR.
A total of 222 patients were randomized to receive ALUNBRIG either 90 mg once daily (90 mg arm; n=112) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (90→180 mg arm; n=110). Randomization was stratified by CNS metastases (present vs absent) and best prior response to crizotinib (complete or partial response vs any other response/unevaluable).
Baseline demographic characteristics of the overall study population were: median age 54 years (range 18 to 82, 23% 65 and over), 67% White and 31% Asian, 57% female, 36% ECOG PS 0 and 57% ECOG PS 1, and 95% never or former smokers. The disease characteristics of the overall study population were: Stage IV disease in 98%, adenocarcinoma histology in 97%, prior systemic chemotherapy in 74%, CNS metastases in 69% (61% had received prior CNS radiation), bone metastases in 39%, and liver metastases in 26% of patients. Sixty-four percent of patients had an objective response to prior crizotinib.
The median duration of follow-up was 8 months (range: 0.1-20.2). Efficacy results from ALTA are summarized in Table 9.
Table 9. ALTA Efficacy Results:
| Efficacy Parameter | IRC Assessment | Investigator Assessment | ||
|---|---|---|---|---|
| 90 mg once daily (N=112) | 90→180 mg once daily (N=110) | 90 mg once daily (N=112) | 90→180 mg once daily (N=110) | |
| Overall Response Rate (95% CI) | 48% (39,58) | 53% (43,62) | 45% (35,54) | 54% (44,63) |
| Complete Response, n (%) | 4 (3.6%) | 5 (4.5%) | 1 (0.9%) | 4 (3.6%) |
| Partial Response, n (%) | 50 (45%) | 53 (48%) | 49 (44%) | 55 (50%) |
| Duration of Response, median in months (95% CI) | 13.8 (7.4, NE) | 13.8 (9.3, NE) | 13.8 (5.6, 13.8) | 11.1 (9.2, 13.8) |
CI = Confidence Interval; NE = Not Estimable
IRC assessment of intracranial ORR and intracranial DOR according to RECIST v1.1 in the subgroup of 44 patients with measurable CNS metastases (≥10 mm in longest diameter) at baseline are summarized in Table 10. Duration of intracranial response was measured from date of first intracranial response until intracranial disease progression (new lesions, intracranial target lesion diameter growth ≥20% from nadir, or unequivocal progression of intracranial nontarget lesions) or death.
Table 10. Intracranial Overall Response in Patients with Measurable CNS Metastases in ALTA:
| Efficacy Parameter | IRC Assessment | |
|---|---|---|
| 90 mg once daily (N=26) | 90→180 mg once daily (N=18) | |
| Intracranial Overall Response Rate, (95% CI) | 42% (23,63) | 67% (41,87) |
| Complete Response, n (%) | 2 (7.7%) | 0 |
| Partial Response, n (%) | 9 (35%) | 12 (67%) |
| Duration of Intracranial Response | ||
| Number of Responders | 11 | 12 |
| Intracranial Response Duration ≥6 months | 7 (64%) | 6 (50%) |
| Intracranial Response Duration ≥12 months | 4 (36%) | 3 (25%) |
CI = Confidence Interval
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.