ACAM2000 Powder for solution for injection Ref.[10408] Active ingredients: Smallpox, live attenuated
Source: FDA, National Drug Code (US) Revision Year: 2018
1. Indications and Usage
ACAM2000 is indicated for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection.
2. Dosage and Administration
Administer ACAM2000 only after being trained on the safe and effective administration of the vaccine by the percutaneous route (scarification). ACAM2000 should not be injected by the intradermal, subcutaneous, intramuscular, or intravenous route. Provide each patient with the FDA-approved Medication Guide prior to administering the vaccine.
2.1 Instructions for Vaccine Preparation
Reconstitution
ACAM2000 is reconstituted by addition of 0.3 mL of diluent to the vial containing lyophilized vaccine. Note: this 0.3 mL of diluent is not the entire content of the diluent vial. ACAM2000 should only be reconstituted with 0.3 mL of the diluent provided. The vaccine vial should be removed from cold storage and brought to room temperature before reconstitution. The flip cap seals of the vaccine and diluent vials are removed, and each rubber stopper is wiped with an isopropyl alcohol swab and allowed to dry thoroughly. Using aseptic technique and a sterile 1 mL syringe fitted with a 25 gauge x 5/8" needle (provided), draw up 0.3 mL of diluent and transfer the entire content of the syringe to the vaccine vial. Gently swirl to mix but try not to get product on the rubber stopper. The reconstituted vaccine should be a clear to slightly hazy, colorless to straw-colored liquid free from extraneous matter. Reconstituted vaccine should be inspected visually for particulate matter and discoloration prior to administration. If particulate matter or discoloration is observed, the vaccine should not be used and the vial should be disposed safely [See Preparation/Handling Precautions and Instructions for Disposal (2.2)].
Storage following Reconstitution
After reconstitution, ACAM2000 vaccine may be administered within 6 to 8 hours if kept at room temperature (20-25°C, 68-77°F). Unused, reconstituted ACAM2000 vaccine may be stored in a refrigerator (2-8°C, 36-46°F) up to 30 days, after which it should be discarded as a biohazardous material. [See Preparation / Handling Precautions and Instructions for Disposal (2.2)] Exposure of reconstituted vaccine to room temperature during vaccination sessions should be minimized by placing it in refrigerator or on ice between patient administrations.
2.2 Preparation / Handling Precautions and Instructions for Disposal
Personnel preparing and administering the vaccine should wear surgical or protective gloves and avoid contact of vaccine with skin, eyes or mucous membranes.
The vaccine vial, its stopper, the diluent syringe, the vented needle used for reconstitution, the bifurcated needle used for administration, and any gauze or cotton that came in contact with the vaccine should be discarded in leak-proof, puncture-proof biohazard containers. These containers should then be disposed of appropriately.
2.3 Vaccination Instructions
The reconstituted vaccine should be brought to room temperature prior to administration. Before administration, the vial contents should be visually examined to verify the absence of particulates and gently swirled, without allowing the product to contact the rubber stopper, if necessary to re-dissolve any precipitates that might have formed.
The site of vaccination is the upper arm over the insertion of the deltoid muscle.
No skin preparation should be performed unless the skin at the intended site of vaccination is obviously dirty, in which case an alcohol swab(s) may be used to clean the area. If alcohol is used, the skin must be allowed to dry thoroughly to prevent inactivation of the live vaccine virus by the alcohol.
Remove the vaccine vial cap. Remove bifurcated needle from individual wrapping. Submerge bifurcated end of needle in reconstituted vaccine solution. The needle will pick up a droplet of vaccine (0.0025 mL) within the fork of the bifurcation. Use aseptic technique, i.e., do not insert the upper part of the needle that has been in contact with fingers into the vaccine vial, and never re‑dip the needle into the vaccine vial if the needle has touched skin.
Deposit the droplet of vaccine onto clean, dry skin of the arm prepared for vaccination. The needle is held between thumb and first finger perpendicular to the skin. The wrist of the hand holding the needle of the vaccinator rests against the patient’s arm. Rapidly make 15 jabs of the needle perpendicular to the skin through the vaccine droplet to puncture the skin, within a diameter of about 5 mm. The jabs should be vigorous enough so that a drop of blood appears at the vaccination site.
Any excess droplets of vaccine and blood should be wiped off the skin using a dry gauze pad and discarded in a biohazard container. Discard the needle in a biohazard sharps container. Close the vaccine vial by reinserting the rubber cap and return to a refrigerator or place on ice unless it will be used immediately to vaccinate another subject. [See Storage Following Reconstitution (2.1.2).]
Cover the vaccination site loosely with a gauze bandage, using first aid adhesive tape to keep it in place. This bandage provides a barrier to protect against spread of the vaccinia virus. If the vaccinee is involved in direct patient care, the gauze should be covered with a semipermeable (semiocclusive) dressing as an additional barrier. A semipermeable dressing is one that allows for the passage of air but does not allow for the passage of fluids.
Wash hands with soap and warm water or with alcohol-based hand rubs such as gels or foams after direct contact with the vaccination site, the bandage or clothes, towels or sheets that might be contaminated with virus from the vaccination site. This is vital in order to remove any virus from your hands and prevent contact spread.
Put the contaminated bandages in a sealed plastic bag and throw them away in the trash.
Wash separately clothing, towels, bedding or other items that may have come in direct contact with the vaccination site or drainage from the site, using hot water with detergent and/or bleach. Wash hands afterwards.
Don’t use a bandage that blocks air from the vaccination site. This may cause the skin at the vaccination site to soften and wear away. Use loose gauze secured with medical tape to cover the site.
Don’t put salves or ointments on the vaccination site.
2.4 Instructions for Interpreting Vaccination Response
Primary Vaccinees
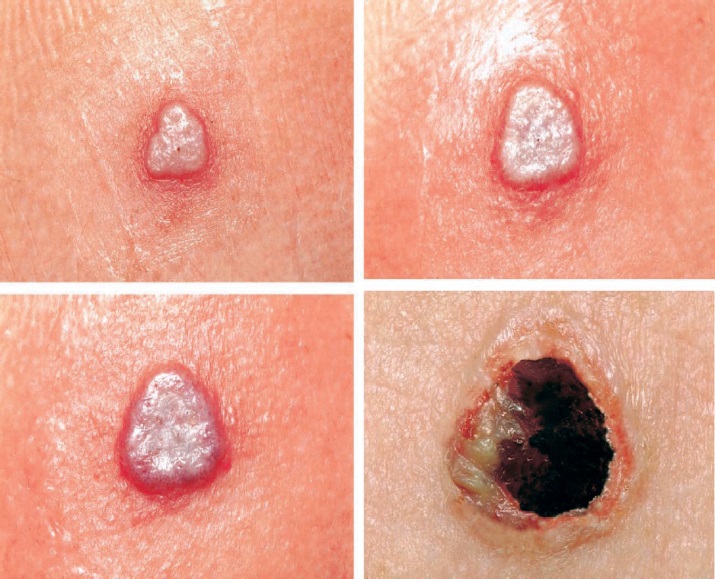
In an individual vaccinated for the first time (primary vaccination), the expected response to vaccination is the development of a major cutaneous reaction (characterized by a pustule) at the site of inoculation. The lesion evolves gradually, with appearance of a papule at the site of vaccination after 2-5 days. The papule becomes vesicular, then pustular, and reaches its maximum size at 8-10 days after vaccination. The pustule dries and forms a scab, which usually separates within 14-21 days, leaving a pitted scar. (See Figure 1) Formation of a major cutaneous reaction by day 6-8 is evidence of a successful ‘take’ and acquisition of protective immunity. An equivocal reaction is any reaction that is not a major reaction, and indicates a non-take (vaccination failure) due to impotent vaccine or inadequate vaccination technique.
Previously Vaccinated Individuals (Revaccination)
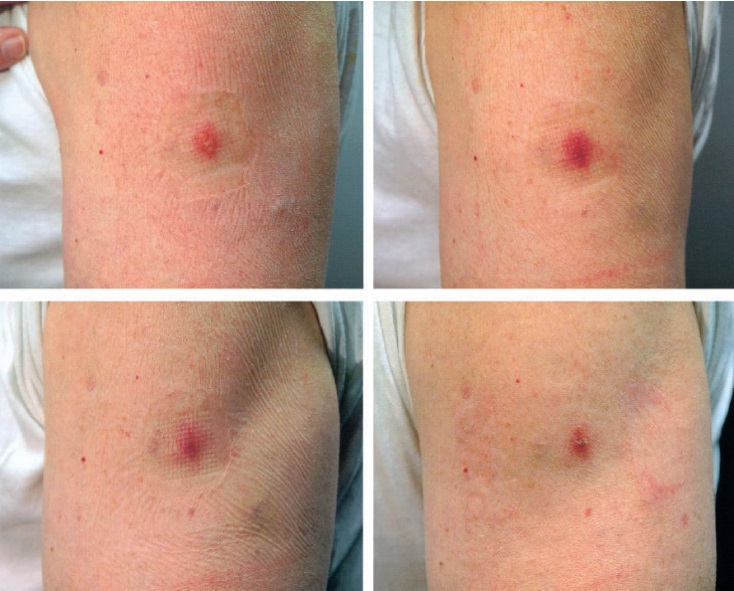
Successful vaccination in an individual previously exposed to vaccine is confirmed when a major cutaneous reaction [See Primary Vaccinees (2.4.1) and Figure 1] is observed 6 to 8 days post-vaccination. However any prior vaccination may modify (reduce) the cutaneous response upon revaccination (Figure 2) such that the absence of a cutaneous response does not necessarily indicate vaccination failure. Previously vaccinated individuals who do not have a cutaneous response on revaccination do not require revaccination to try to elicit a cutaneous response.
Vaccination Failures
Individuals who are not successfully vaccinated (i.e., vaccination failures) after primary vaccination may be revaccinated again in an attempt to achieve a satisfactory take. The vaccination procedures should be checked, and vaccination repeated with vaccine from another vial or vaccine lot, employing the same technique described in 2.3 [see Vaccination Instructions (2.3)].
If a repeat vaccination is conducted using vaccine from another vial or vaccine lot fails to produce a major reaction, healthcare providers should consult the Centers for Disease Control and Prevention (CDC) Emergency Operations Center (EOC) at 770-488-7100 and their state or local health department before giving another vaccination.
Figure 1. Progression of major cutaneous reaction after primary vaccination1:
Day 5 Day 8
Day 10 Day 14
Figure 2: Progression of major cutaneous reaction after revaccination1:
Day 3 Day 7
Day 10 Day 14
2.5 Booster Schedule
Persons at continued high risk of exposure to smallpox (e.g., research laboratory workers handling variola virus) should receive repeat ACAM2000 vaccination every three years.
2.6 Smallpox Vaccination Recommendations from US Government Agencies
Additional information may be obtained from U.S. Department of Defense and U.S. Centers for Disease Control and Prevention (CDC) about smallpox vaccination.
16.2. Storage and Handling
ACAM2000 should be stored in a freezer with an average temperature of -15°C to -25°C (+5°F to -13°F).
Prior to reconstitution, ACAM2000 vaccine retains a potency of 1.0 × 108 PFU or higher per dose for at least 18 months when stored at refrigerated temperatures of +2-8°C (36-46°F).
During shipment, ACAM2000 should be maintained at a temperature of ‑10°C or colder.
After reconstitution, ACAM2000 vaccine may be administered during a 6 to 8 hour workday at room temperature (20-25°C, 68-77°F). Reconstituted ACAM2000 vaccine may be stored in a refrigerator (2-8°C, 36-46°F) no longer than 30 days, after which it should be discarded [see Dosage and Administration (2.3)]. Diluent for Smallpox Vaccine, (Vero Cells) Lyophilized, ACAM2000 should be stored at room temperature (15-30°C, 59-86°F).
ACAM2000 contains live vaccinia virus that is transmissible, and should be handled as an infectious agent once vials are open. See 2.1 [Instructions for Vaccine Preparation] and 2.2 [Preparation/Handling Precautions and Instructions for Disposal] for details on handling and disposal.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.