ACANYA Gel Ref.[50737] Active ingredients: Benzoyl peroxide Clindamycin
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
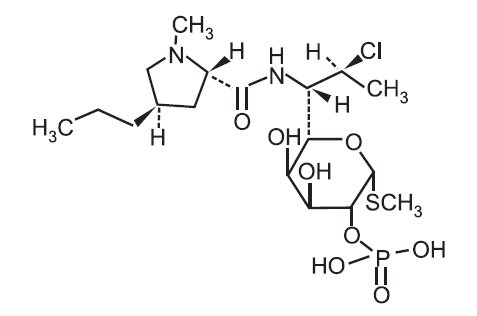
ACANYA (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/2.5% is a combination product with two active ingredients in a white to off-white, opaque, smooth, aqueous gel formulation intended for topical use. Clindamycin phosphate is a water-soluble ester of the semisynthetic antibiotic produced by a 7(S)-chloro-substitution of the 7==(R)==‑hydroxyl group of the parent antibiotic lincomycin.
The chemical name for clindamycin phosphate is Methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate). The structural formula for clindamycin phosphate is represented below:
Clindamycin phosphate:
Molecular Formula: C18H34ClN2O8PS Molecular Weight: 504.97
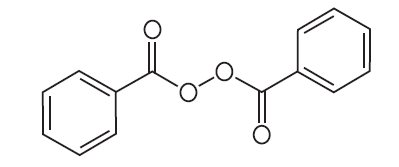
Benzoyl peroxide is an antibacterial and keratolytic agent. The structural formula for benzoyl peroxide is represented below:
Benzoyl peroxide:
Molecular Formula: C14H10O4 Molecular Weight: 242.23
ACANYA Gel contains the following inactive ingredients: carbomer 980, potassium hydroxide, propylene glycol, and purified water. Each gram of ACANYA Gel contains 1.2% of clindamycin phosphate which is equivalent to 1% clindamycin.
| Dosage Forms and Strengths |
|---|
|
Gel, 1.2%/2.5%. Each gram of ACANYA Gel contains 10 mg (1%) clindamycin as phosphate, and 25 mg (2.5%) benzoyl peroxide in a white to off-white, opaque, smooth gel. |
| How Supplied |
|---|
|
ACANYA Gel, 1.2%/2.5%, a white to off-white smooth gel is supplied as: NDC 13548-132-50 50 g pump. Dispensing Instructions for the Pharmacist
Distributed by: Bausch Health US, LLC, Bridgewater, NJ 08807 USA Manufactured by: Bausch Health Companies Inc., Laval, Quebec H7L 4A8, Canada |
Drugs
| Drug | Countries | |
|---|---|---|
| ACANYA | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.