ACCOLATE Coated tablet Ref.[50354] Active ingredients: Zafirlukast
Source: FDA, National Drug Code (US) Revision Year: 2015
Product description
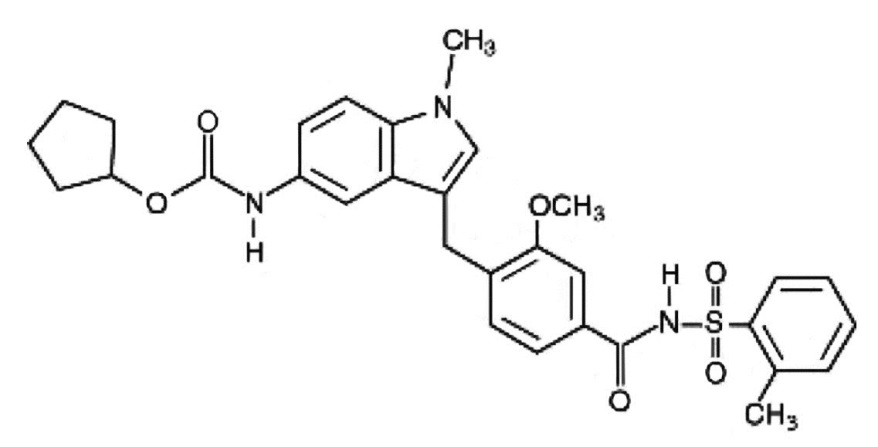
Zafirlukast is a synthetic, selective peptide leukotriene receptor antagonist (LTRA), with the chemical name 4-(5-cyclopentyloxy-carbonylamino-1-methyl-indol-3-ylmethyl)-3-methoxy-N-o-tolylsulfonylbenzamide. The molecular weight of zafirlukast is 575.7 and the structural formula is:
The empirical formula is: C31H33N3O6S
Zafirlukast, a fine white to pale yellow amorphous powder, is practically insoluble in water. It is slightly soluble in methanol and freely soluble in tetrahydrofuran, dimethylsulfoxide, and acetone.
ACCOLATE is supplied as 10 and 20 mg tablets for oral administration.
Inactive Ingredients: Film-coated tablets containing croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose, povidone, hypromellose, and titanium dioxide.
| How Supplied |
|---|
|
ACCOLATE 10 mg Tablets, (NDC 49884-589-02) white, round, biconvex, film-coated tablets debossed with "P" on one side and "10" on the other, are supplied in opaque HDPE bottles of 60 tablets. ACCOLATE 20 mg Tablets, (NDC 49884-590-02) white, round, biconvex, film-coated tablets debossed with "P" on one side and "20" on the other, are supplied in opaque HDPE bottles of 60 tablets. ACCOLATE is a registered trademark of Par Pharmaceutical, Inc. Manufactured by: Par Pharmaceutical Companies, Inc., Chestnut Ridge, NY 10977 U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| ACCOLATE | Mexico, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.