ADENOCARD Solution for injection Ref.[10740] Active ingredients: Adenine Deoxy Nucleoside
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
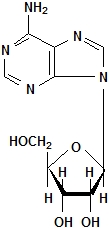
Adenosine is an endogenous nucleoside occurring in all cells of the body. It is chemically 6-amino-9-β-D-ribofuranosyl-9-H-purine and has the following structural formula:
Adenosine is a white crystalline powder. It is soluble in water and practically insoluble in alcohol. Solubility increases by warming and lowering the pH. Adenosine is not chemically related to other antiarrhythmic drugs. Adenocard (adenosine injection) is a sterile, nonpyrogenic solution for rapid bolus intravenous injection. Each mL contains 3 mg adenosine and 9 mg sodium chloride in Water for Injection. The pH of the solution is between 4.5 and 7.5.
The Ansyr plastic syringe is molded from a specially formulated polypropylene. Water permeates from inside the container at an extremely slow rate which will have an insignificant effect on solution concentration over the expected shelf life.
Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the syringe material.
| How Supplied |
|---|
|
Adenocard (adenosine injection) is supplied as a sterile non-pyrogenic solution in normal saline. NDC 0469-8234-12 Product Code 823412 6 mg/2 mL (3 mg/mL) in 2 mL (fill volume) Ansyr plastic disposable syringe, in a package of ten. NDC 0469-8234-14 Product Code 823414 12 mg/4 mL (3 mg/mL) in 4 mL (fill volume) Ansyr plastic disposable syringe, in a package of ten. Marketed by: Astellas Pharma US, Inc., Northbrook, IL 60062 USA Manufactured by: Hospira, Inc., Lake Forest, IL 60045 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| ADENOCARD | Brazil, Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.