ADYNOVI Powder and solvent for solution for injection Ref.[9404] Active ingredients: Rurioctocog alfa pegol
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Baxalta Innovations GmbH, Industriestrasse 67, A-1221, Vienna, Austria
Therapeutic indications
Treatment and prophylaxis of bleeding in patients 12 years and above with haemophilia A (congenital factor VIII deficiency).
Posology and method of administration
Treatment should be under the supervision of a physician experienced in the treatment of haemophilia.
Previously untreated patients
The safety and efficacy of ADYNOVI in previously untreated patients have not yet been established.
No data are available.
Treatment monitoring
During the course of treatment, appropriate determination of factor VIII levels is advised to guide the dose to be administered and the frequency of repeated infusions. Individual patients may vary in their response to factor VIII, demonstrating different half-lives and recoveries. Dose based on bodyweight may require adjustment in underweight or overweight patients. In the case of major surgical interventions in particular, precise monitoring of the substitution therapy by means of coagulation analysis (plasma factor VIII activity) is indispensable.
A field study has indicated that plasma factor VIII levels can be monitored using either a chromogenic substrate assay or a one stage clotting assay routinely used in clinical laboratories.
Posology
The dose and duration of the substitution therapy depend on the severity of the factor VIII deficiency, on the location and extent of the bleeding and on the patient’s clinical condition.
The number of units of factor VIII administered is expressed in International Units (IU), which are related to the current WHO concentrate standard for factor VIII products. Factor VIII activity in plasma is expressed either as a percentage (relative to normal human plasma) or preferably in International Units (relative to an International Standard for factor VIII in plasma).
One International Unit (IU) of factor VIII activity is equivalent to that quantity of factor VIII in one ml of normal human plasma.
On demand treatment
The calculation of the required dose of factor VIII is based on the empirical finding that 1 IU factor VIII per kg body weight raises the plasma factor VIII activity by 2 IU/dl. The required dose is determined using the following formula:
Required international units (IU) = body weight (kg) x desired factor VIII rise (%) x 0.5
The amount to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case.
In the case of the following haemorrhagic events, factor VIII activity should not fall below the given plasma activity level (in % of normal or IU/dl) in the corresponding period.
The following Table 1 can be used to guide dosing in bleeding episodes and surgery.
Table 1. Guide for dosing in bleeding episodes and surgery:
| Degree of haemorrhage/type of surgical procedure | Factor VIII level required (% or IU/dl) | Frequency of doses (hours)/duration of therapy (days) |
|---|---|---|
| Haemorrhage | ||
| Early haemarthrosis, muscle bleeding or oral bleeding. | 20–40 | Repeat injections every 12 to 24 hours. At least 1 day, until the bleeding episode, as indicated by pain, is resolved or healing is achieved. |
| More extensive haemarthrosis, muscle bleeding or haematoma | 30–60 | Repeat injections every 12 to 24 hours for 3–4 days or more until pain and acute disability are resolved. |
| Life threatening haemorrhages. | 60–100 | Repeat injections every 8 to 24 hours until threat is resolved. |
| Surgery | ||
| Minor Including tooth extraction. | 30–60 | Every 24 hours at least 1 day, until healing is achieved. |
| Major | 80–100 (pre- and postoperative) | Repeat injections every 8 to 24 hours until adequate wound healing, then continue therapy for at least another 7 days to maintain a factor VIII activity of 30% to 60% (IU/dl). |
Prophylaxis
For long term prophylaxis, the recommended dose is 40 to 50 IU of ADYNOVI per kg bodyweight twice weekly in 3 to 4 day intervals. Adjustments of doses and administration intervals may be considered based on achieved FVIII levels and individual bleeding tendency (see section 5.2).
Paediatric population
On demand treatment dosing in paediatric patients (12 to 18 years of age) is the same as for adult patients. Prophylactic treatment for patients from 12 to <18 years is the same as for adult patients. The long-term safety of ADYNOVI in children below 12 years has not yet been established. Adjustments of doses and administration intervals may be considered based on achieved FVIII levels and individual bleeding tendency (see section 5.2).
Method of administration
ADYNOVI is for intravenous use. The rate of administration should be determined to ensure the comfort of the patient up to a maximum of 10 ml/min.
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Overdose
No symptoms of overdose with recombinant coagulation factor VIII have been reported.
Shelf life
Shelf life
Unopened vial: 2 years.
Before opening the product may be stored at room temperature (up to 30°C) for a period of up to 3 months. The end of the 3-month storage at room temperature should be recorded on the product carton. This date should never exceed the one initially mentioned on the outer carton. At the end of this period the product shall not be put back in the refrigerator, but shall be used or discarded.
After reconstitution: Chemical and physical in-use stability has been demonstrated for 3 hours at a temperature not above 30°C. From a microbiological point of view, unless the method of reconstitution precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user. Do not refrigerate.
Special precautions for storage
Store refrigerated (2° to 8°C).
Do not freeze.
ADYNOVI with BAXJECT II Hi-Flow device: Keep the vial in the outer carton in order to protect from light.
ADYNOVI in BAXJECT III system: Keep the sealed blister in the outer carton in order to protect from light. For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
Type I glass vial, closed with a chlorobutyl rubber stopper, containing 250 IU, 500 IU, 1000 IU or 2000 IU of powder.
Type I glass vial, closed with a chlorobutyl rubber stopper, containing 5 ml of sterilised water for injections.
The medicinal product is provided in one of the following configurations:
- ADYNOVI with BAXJECT II Hi-Flow device: Each pack contains a powder vial, a solvent vial and a device for reconstitution (BAXJECT II Hi-Flow).
- ADYNOVI in BAXJECT III system: Each pack contains a ready to use BAXJECT III system in a sealed blister, withthe powder vial and the solvent vial preassembled for reconstitution.
Special precautions for disposal and other handling
The reconstituted medicinal product should be inspected visually for particulate matter and discoloration prior to administration. The solution should be clear or slightly opalescent. Solutions that are cloudy or have deposits should not be used.
After reconstitution, the solution has a pH of 6.7 to 7.3. The osmolality is ≥380 mOsmol/kg.
Preparation and reconstitution using the BAXJECT II Hi-Flow device
For reconstitution use only the solvent vial and the reconstitution device provided in the pack.
- Use antiseptic technique (clean and low-germ conditions) and a flat work surface during the reconstitution procedure.
- Allow the vials of powder and solvent to reach room temperature (between 15°C and 25°C) before use.
- Remove plastic caps from the powder and solvent vials.
- Clean rubber stoppers with an alcohol wipe and allow to dry prior to use.
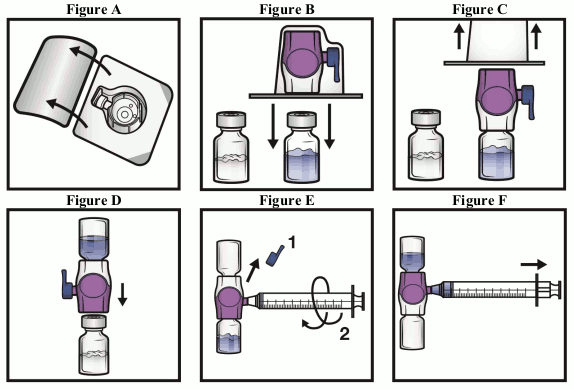
- Open the BAXJECT II Hi-Flow device package by peeling away the lid, without touching the inside (Figure A). Do not remove the device from the package.
- Turn the package over. Press straight down to fully insert the clear plastic spike through the solvent vial stopper (Figure B).
- Grip the BAXJECT II Hi-Flow package at its edge and pull the package off the device (Figure C). Do not remove the blue cap from the BAXJECT II Hi-Flow device. Do not touch the exposed purple plastic spike.
- Turn the system over so that the solvent vial is on top. Quickly insert the purple plastic spike fully into the powder vial stopper by pushing straight down (Figure D). The vacuum will draw the solvent into the powder vial.
- Swirl gently until the powder is completely dissolved. Do not refrigerate after reconstitution.
Administration
- Visually inspect the reconstituted solution for particulate matter and discoloration prior to administration.
- The appearance of the reconstituted solution is clear and colourless.
- Do not use if particulate matter or discoloration is observed.
- Administer as soon as possible, but no later than 3 hours after reconstitution.
Administration Steps:
- Remove the blue cap from the BAXJECT II Hi-Flow device (Figure E). Do not draw air into the syringe. Connect the syringe to the BAXJECT II Hi-Flow. Use of a Luer-lock syringe is recommended.
- Turn the system upside down (powder vial now on top). Draw the reconstituted solution into the syringe by pulling the plunger back slowly (Figure F).
- Disconnect the syringe; attach a suitable needle and inject intravenously. If a patient is to receive more than one vial of ADYNOVI, the contents of multiple vials may be drawn into the same syringe. A separate BAXJECT II Hi-Flow device is required to reconstitute each vial of ADYNOVI with the solvent.
- Administer over a period of up to 5 minutes (maximum infusion rate 10 ml per min).
It is strongly recommended that every time ADYNOVI is administered, the name and batch number of the product are recorded. Peel-off labels are provided on the powder vial.
Reconstitution with the BAXJECT III system
Do not use if the lid is not completely sealed on the blister
- If the product is still stored in a refrigerator, take the sealed blister (contains powder and solvent vials preassembled with the system for reconstitution) from the refrigerator and let it reach room temperature (between 15 °C and 25 °C).
- Wash your hands thoroughly using soap and warm water.
- Open the ADYNOVI blister by peeling away the lid. Remove the BAXJECT III system from the blister.
- Place the powder vial on a flat surface with the solvent vial on top (Figure 1). The solvent vial has a blue stripe. Do not remove the blue cap until instructed in a later step.
- With one hand holding the powder vial in the BAXJECT III system, press down firmly on the solvent vial with the other hand until the system is fully collapsed and the solvent flows down into the powder vial (Figure 2). Do not tilt the system until the transfer is complete.
- Verify that the solvent transfer is complete. Swirl gently until all material is dissolved (Figure 3). Be sure that the powder is completely dissolved, otherwise not all reconstituted solution will pass through the device filter. The product dissolves rapidly (usually in less than 1 minute). After reconstitution the solution should be clear, colourless and free from particles.
Administration
- Visually inspect the reconstituted solution for particulate matter and discoloration prior to administration.
- The appearance of the reconstituted solution is clear and colourless.
- Do not use if particulate matter or discoloration is observed.
- Administer as soon as possible, but no later than 3 hours after reconstitution.
Administration Steps:
- Remove the blue cap from the BAXJECT III device. Do not draw air into the syringe. Connect the syringe to the BAXJECT III device. Use of a Luer-lock syringe is recommended.
- Turn the system upside down (powder vial now on top). Draw the reconstituted solution into the syringe by pulling the plunger back slowly.
- Disconnect the syringe; attach a suitable needle and inject intravenously. If a patient is to receive more than one vial of ADYNOVI, the contents of multiple vials may be drawn into the same syringe.
- Administer over a period of up to 5 minutes (maximum infusion rate 10 ml per min).
It is strongly recommended that every time ADYNOVI is administered, the name and batch number of the product are recorded. Peel-off labels are provided on the blister.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.