AETHOXYSKLEROL Solution for injection Ref.[27688] Active ingredients: Polidocanol
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Ferndale Pharmaceuticals Ltd, Unit 740, Thorp Arch Estate, Wetherby, West Yorkshire, LS23 7FX
4.1. Therapeutic indications
Aethoxysklerol is indicated for sclerotherapy of varicose veins of the lower extremities.
4.2. Posology and method of administration
Different concentrations of Aethoxysklerol are required, depending on the type and size of the varicose veins to be treated.
If several concentrations are stated for a certain type of vein (see table below), the diameter of the vein and the patient’s individual situation should be considered. In case of doubt the lower concentration should be chosen.
Depending on the degree and extent of the varicose veins, several treatments may be required.
| Sclerotherapy of | Aethoxysklerol concentration | |||||
| 2.5 mg/ml | 5 mg/ml | 10 mg/ml | 20 mg/ml | 30 mg/ml | ||
| Telangiectasias (spider veins) | ● | ● | Liquid | |||
| Microfoam | ||||||
| Central veins of telangiectasias | ● | ● | ● | Liquid | ||
| Microfoam | ||||||
| Reticular veins | ● | Liquid | ||||
| Microfoam | ||||||
| Small varicose veins | ● | Liquid | ||||
| ● | Microfoam | |||||
| Medium-sized varicose veins | ● | ● | Liquid | |||
| ● | ● | Microfoam | ||||
| Large varicose veins | ● | Liquid | ||||
| ● | Microfoam | |||||
Posology
Sclerotherapy of varicose veins
Generally, the dose of 2 mg lauromacrogol 400 per kg body weight per day should not be exceeded.
For a patient weighing 70 kg, a total of up to 140 mg lauromacrogol 400 can be injected. 140 mg lauromacrogol 400 are contained in:
| Aethoxysklerol | 2.5 mg/ml | 5 mg/ml | 10 mg/ml | 20 mg/ml | 30 mg/ml |
| 140 mg lauromacrogol 400 | 56 ml | 28 ml | 14 ml | 7 ml | 4.6 ml |
When applying as a sclerosing microfoam, it is recommended not to exceed the total dose of 10 ml microfoam (the sum of the liquid and air components) per session and day – irrespective of the patient’s body weight and concentration of lauromacrogol 400.
Sclerotherapy of telangiectasias
Depending on the size of the area to be treated, per puncture 0.1-0.2 ml Aethoxysklerol 2.5 mg/ml or 5 mg/ml are injected intravenously.
Sclerotherapy of central veins of telangiectasias
Depending on the size of the area to be treated, per puncture 0.1-0.2 ml Aethoxysklerol 2.5 mg/ml, 5 mg/ml or 10 mg/ml are injected intravenously.
Sclerotherapy of reticular veins
Depending on the size of the varicose vein to be treated, per puncture 0.1-0.3 ml Aethoxysklerol 10 mg/ml are injected intravenously.
Sclerotherapy of small varicose veins
Depending on the size of the varicose vein to be treated, per puncture 0.1-0.3 ml liquid Aethoxysklerol 10 mg/ml are injected intravenously.
When using Aethoxysklerol 10 mg/ml microfoam, e.g. for the treatment of tributary varicose veins (collateral varices), up to 4-6 ml are injected per puncture. When treating perforating veins with microfoam up to 2-4 ml are injected per puncture.
Sclerotherapy of medium-sized varicose veins
Depending on the diameter of the varicose veins to be treated, Aethoxysklerol 20 mg/ml or 30 mg/ml is used.
Depending on the length of the segment to be treated, several injections with up to 2 ml of liquid Aethoxysklerol 20 mg/ml or 30 mg/ml per injection may be administered, without exceeding the maximum daily dose.
When using Aethoxysklerol 20 mg/ml microfoam, e.g. for the treatment of perforating or tributary varicose veins, up to 2 ml microfoam are injected per puncture. When using Aethoxysklerol 20 mg/ml or 30 mg/ml microfoam, e.g. for the treatment of the saphenous veins, up to 4 ml are injected per puncture for the small saphenous veins and up to 6 ml for the great saphenous veins.
Sclerotherapy of large varicose veins
Depending on the length of the segment to be treated, several injections with up to 2 ml of liquid Aethoxysklerol 30 mg/ml per injection may be administered, without exceeding the maximum daily dose.
When using Aethoxysklerol 30 mg/ml microfoam, e.g. for the treatment of the saphenous veins, up to 4 ml are injected per puncture for the small saphenous veins and up to 6 ml for the great saphenous veins.
Elderly population
No specific dose recommendations apply.
Paediatric population
There is no relevant use of Aethoxysklerol in the paediatric population in children or adolescents for the indication of sclerotherapy of varicose veins of the lower extremities.
Hepatic impairment / Renal impairment
No pharmacokinetic studies have been performed in patients with hepatic or renal impairment. The use of sclerotherapy should be cautious and assessed in patients with moderate hepatic or renal impairment, in whom the treatment benefit clearly outweighs the risk. Aethoxysklerol is not recommended for use in patients with severe hepatic or renal impairment.
Method of administration
Sclerotherapy of varicose veins
All injections must be given intravenously; the position of the needle should be checked (e.g. by aspiration of blood).
Strict aseptic technique must be maintained while handling Aethoxysklerol.
Aethoxysklerol is a single-use parenteral product. Once the container is opened, use immediately and discard any unused portion.
Visually inspect for particulate matter before use. Solutions that contain particulate matter should not be used.
Additionally for microfoam administration refer to the detailed instructions in section 6.6.
Sclerotherapy of telangiectasias
Sclerotherapy of central veins of telangiectasias
Sclerotherapy of reticular veins
Injections are usually carried out in a leg placed horizontally. Smooth-moving disposable syringes are used.
For telangiectasias very fine needles (e.g. insulin needles) are used. The puncture is carried out tangentially and the injection given slowly.
Sclerotherapy of small, medium-sized and large varicose veins
Irrespective of the mode of venepuncture (in a standing patient with the cannula only or in a sitting patient with a syringe ready for injection), injections are usually carried out in a leg placed horizontally.
Smooth-moving disposable syringes are recommended for sclerotherapy as well as needles with different diameters, depending on the indication.
When using microfoam, the leg can be placed horizontally or elevated approx. 30–45° above the horizontal for injection. Direct puncture and injection into non-visible veins should be guided by duplex ultrasound. The needle should not be smaller than 25G.
Note: Thrombi, which occasionally develop, are removed by stab incision and thrombus expression.
Compression treatment after injection of Aethoxysklerol
Once the injection site has been covered, a tight compression bandage or elastic stocking should be applied. After that, the patient should walk for 30 minutes, preferably within reach of the practice.
After sclerotherapy with liquid Aethoxysklerol, compression is applied immediately.
After sclerotherapy with microfoam the patient’s leg is initially immobilised for 2-5 minutes. Valsalva’s manoeuvre and muscle activation should be avoided during this time. Compression should not be applied immediately but 5 to 10 minutes after injection.
Compression should be applied for a few days up to several weeks, depending on the extent and severity of the varicose veins.
To make sure the bandage does not slip, especially on the thigh and conical limbs, a foam bandage support under the actual compression bandage is recommended.
4.9. Overdose
Local overdose (caused by the injected volume or concentration being too high) may cause local necrosis, especially after extravenous injection.
For management of local toxicity after improper administration when treating varicose veins refer to section 4.4 above.
6.3. Shelf life
3 years.
The ampoule is intended for single use. After first opening, the medicinal product should be used immediately. Any residual amount must be discarded.
6.4. Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5. Nature and contents of container
2ml ampoule (Type I glass):
| Ampoule identification Stripe colours & format | |
| Aethoxysklerol 2.5 mg/ml | Two red |
| Aethoxysklerol 5 mg/ml | Two white and one red |
| Aethoxysklerol 10 mg/ml | One yellow and one red |
| Aethoxysklerol 20 mg/ml | One green and one red |
| Aethoxysklerol 30 mg/ml | One blue and one red and one white |
6.6. Special precautions for disposal and other handling
No special requirements for disposal.
Preparation of the Microfoam
Preparation of the microfoam using the Tessari and Dual Syringe System (DSS) techniques, respectively, is described below. Other suitable techniques may also be used.
The foam must be prepared just before use and administered by a physician appropriately trained in the correct generation and administration of foam.
Strict aseptic technique must be maintained while manufacturing the foam.
The quality of microfoam depends on specific criteria:
a) Concentration of lauromacrogol 400: In order to obtain a very fine-bubbled and stable microfoam, a concentration of 10-30 mg/ml must be used.
b) Proportion of liquid to gas: In general, this proportion is 1 volume of liquid for 4 volumes of gas.
c) Macroscopic appearance: Observe the macroscopic appearance of the microfoam in the syringe: It must be homogenous and fine-bubbled. No unmixed liquid or gas should be visible.
d) Maximum time between preparation and injection: Inject the microfoam soon after preparation (within 60 seconds).
Filling of the syringes for both foam preparation methods
Note: Syringes containing siliconized components produce a less stable foam and their use should be minimised. As two sterile syringes are needed to create the foam, only the second syringe should have a rubber plunger as this will aid a smooth injection.
To create the foam 2 ml of liquid sclerosant is drawn into the first syringe (without a rubber plunger). The second syringe (with a rubber plunger) is fixed to a 0.2 µm sterile filter and 8 ml of sterile air is drawn up.
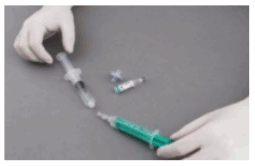
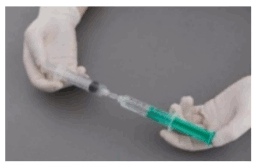
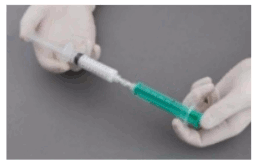
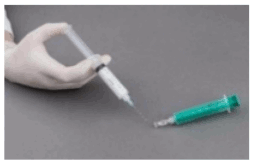
Preparation of sclerosing microfoam with Tessari technique:
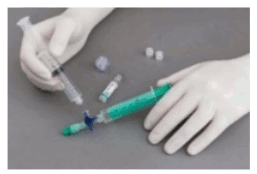
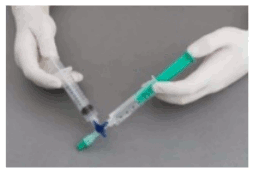
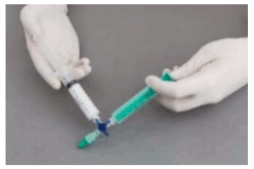
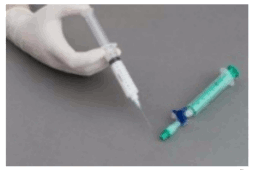
The syringes are firmly connected to a sterile three-way tap/valve (Fig. 1). Foam generation is performed by mixing sclerosant and the air by moving the plungers of both syringes completely forward and backward approximately 20 times under high pressure on both syringes (Fig. 2 and 3). A smooth, consistent foam is obtained. The syringe with the rubber plunger is filled with foam and is then removed from the three-way valve. The vein is injected immediately (Fig. 4).
Figure 1:
Figure 2:
Figure 3:
Figure 4:
Preparation of sclerosing microfoam with DSS (Double Syringe System):
The syringes are firmly connected to a sterile Luer Lock female-female adapter (Fig. 5). Foam generation is performed by mixing sclerosant and the air by moving the plungers of both syringes completely forward and backward 5 times with a short, firm thumb pressure of both hands, so that the pumping must be done against a resistance (Fig. 6 and 7). This is followed by 7 quick forward and backward movements without additional pressure to get a homogenous foam. The syringe with the rubber plunger is filled with foam and is then removed from the adapter. The vein is injected immediately (Fig. 8).
Figure 5:
Figure 6:
Figure 7:
Figure 8:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.