AFSTYLA Powder and solvent for solution for injection Ref.[9432] Active ingredients: Lonoctocog alfa
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: CSL Behring GmbH, Emil-von-Behring-Str. 76, 35041, Marburg, Germany
Therapeutic indications
Treatment and prophylaxis of bleeding in patients with haemophilia A (congenital factor VIII deficiency).
AFSTYLA can be used for all age groups.
Posology and method of administration
Treatment should be under the supervision of a physician experienced in the treatment of haemophilia.
Treatment monitoring
During the course of treatment, appropriate determination of factor VIII levels is advised to guide the dose to be administered and the frequency of repeated injections. Individual patients may vary in their responses to factor VIII, demonstrating different half-lives and recoveries. Dose based on bodyweight may require adjustment in underweight or overweight patients. In the case of major surgical interventions in particular, precise monitoring of the substitution therapy by means of coagulation analysis (plasma factor VIII activity) is indispensable.
When using an in vitro thromboplastin time (aPTT)-based one stage clotting assay for determining factor VIII activity in patients' blood samples, plasma factor VIII activity results can be significantly affected by both the type of aPTT reagent and the reference standard used in the assay. Also there can be significant discrepancies between assay results obtained by aPTT-based one stage clotting assay and the chromogenic assay according to Ph. Eur. This is of importance particularly when changing the laboratory and/or reagents used in the assay.
Plasma factor VIII activity in patients receiving AFSTYLA using either the chromogenic assay or the one-stage clotting assay should be monitored to guide the dose administered and the frequency of repeat injections. The chromogenic assay result most accurately reflects the clinical hemostatic potential of AFSTYLA and is preferred. The one-stage clotting assay result underestimates the factor VIII activity level compared to the chromogenic assay result by approximately 45%. If the one-stage clotting assay is used, multiply the result by a conversion factor of 2 to determine the patient's factor VIII activity level.
Posology
The dose and duration of the substitution therapy depend on the severity of the factor VIII deficiency, on the location and extent of the bleeding and on the patient's clinical condition.
The number of units of factor VIII administered is expressed in International Units (IU), which are related to the current WHO concentrate standard for factor VIII products. Factor VIII activity in plasma is expressed either as a percentage (relative to normal human plasma) or preferably in International Units (relative to an International Standard for factor VIII in plasma).
One International Unit (IU) of factor VIII activity is equivalent to that quantity of factor VIII in one ml of normal human plasma.
Potency assignment is determined using a chromogenic substrate assay. Plasma factor VIII levels can be monitored using either a chromogenic substrate assay or a onestage clotting assay.
On demand treatment
The calculation of the required dose of factor VIII is based on the empirical finding that 1 International Unit (IU) factor VIII per kg body weight raises the plasma factor VIII activity by 2 IU/dl. The required dose is determined using the following formula:
Dose (IU) = body weight (kg) x Desired factor VIII rise (IU/dl or % of normal) x 0.5 (IU/kg per IU/dl)
The amount to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case.
In the case of the following haemorrhagic events, the factor VIII activity should not fall below the given plasma activity level (in % of normal or IU/dl) within the corresponding period. The following table can be used to guide dosing in bleeding episodes and surgery:
| Degree of haemorrhage / Type of surgical procedure | Factor VIII level required (%) (IU/dl) | Frequency of doses (hours) / Duration of therapy (days) |
|---|---|---|
| Haemorrhage | ||
| Early haemarthrosis, muscle bleeding or oral bleeding | 20-40 | Repeat injection every 12 to 24 hours. At least 1 day, until the bleeding episode as indicated by pain is resolved or healing is achieved. |
| More extensive haemarthrosis, muscle bleeding or haematoma | 30-60 | Repeat injection every 12 to 24 hours for 3-4 days or more until pain and acute disability are resolved. |
| Life threatening haemorrhages | 60-100 | Repeat injection every 8 to 24 hours until threat is resolved. |
| Surgery | ||
| Minor surgery including tooth extraction | 30-60 | Inject every 24 hours, at least 1 day, until healing is achieved. |
| Major surgery | 80-100 (pre- and postoperative) | Repeat injection every 8 to 24 hours until adequate wound healing, then therapy for at least another 7 days to maintain a factor VIII activity of 30% to 60% (IU/dl). |
Prophylaxis
The recommended starting regimen is 20 to 50 IU/kg of AFSTYLA administered 2 to 3 times weekly. The regimen may be adjusted based on patient response.
Paediatric population
The recommended starting regimen in children (0 to <12 years of age) is 30 to 50 IU per kg of AFSTYLA administered 2 to 3 times weekly. More frequent or higher doses may be required in children <12 years of age to account for the higher clearance in this age group.
For adolescents of 12 years of age and above, the dose recommendations are the same as for adults (please refer to section 5.2).
Elderly
Clinical studies of AFSTYLA did not include subjects over 65 years of age.
Method of administration
Intravenous use.
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
The reconstituted preparation should be injected slowly at a rate comfortable for the patient at a maximum injection rate of 10 ml/min.
Overdose
In a completed clinical trial a patient who received more than double the prescribed dose of AFSTYLA experienced dizziness, feeling hot, and itching not considered related to AFSTYLA but more plausibly attributed to co-administration of an analgesic.
Shelf life
Shelf life: 3 years.
After reconstitution the chemical and physical in-use stability has been demonstrated for 48 hours at room temperature (below 25°C). From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are in the responsibility of the user.
Special precautions for storage
Store in a refrigerator (2°C to 8°C).
Do not freeze. Keep vials in the outer carton in order to protect from light.
AFSTYLA may be stored at room temperature, not to exceed 25°C, for a single period of up to 3 months, within the expiration date printed on the carton and vial labels. Once the product has been taken out of the refrigerator, the product must not be returned to the refrigerator. Please record the beginning of storage at room temperature on the product carton.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
AFSTYLA 250 IU powder and solvent for solution for injection: Powder (250 IU) in a 6 ml vial (type I glass) with a stopper (rubber), an orange disc (plastic), and a green striped cap (aluminium). 2.5 ml of solvent in a vial (type I glass) with a stopper (rubber), a disc (plastic), and a cap (aluminium).
AFSTYLA 500 IU powder and solvent for solution for injection: Powder (500 IU) in a 6 ml vial (type I glass) with a stopper (rubber), a blue disc (plastic), and a green striped cap (aluminium). 2.5 ml of solvent in a vial (type I glass) with a stopper (rubber), a disc (plastic), and a cap (aluminium).
AFSTYLA 1000 IU powder and solvent for solution for injection: Powder (1000 IU) in a 6 ml vial (type I glass) with a stopper (rubber), a green disc (plastic), and a green striped cap (aluminium). 2.5 ml of solvent in a vial (type I glass) with a stopper (rubber), a disc (plastic), and a cap (aluminium).
AFSTYLA 1500 IU powder and solvent for solution for injection: Powder (1500 IU) in a 10 ml vial (type I glass) with a stopper (rubber), a turquoise disc (plastic), and a green striped cap (aluminium). 5 ml of solvent in a vial (type I glass) with a stopper (rubber), a disc (plastic), and a cap (aluminium).
AFSTYLA 2000 IU powder and solvent for solution for injection: Powder (2000 IU) in a 10 ml vial (type I glass) with a stopper (rubber), a purple disc (plastic), and a green striped cap (aluminium). 5 ml of solvent in a vial (type I glass) with a stopper (rubber), a disc (plastic), and a cap (aluminium).
AFSTYLA 2500 IU powder and solvent for solution for injection: Powder (2500 IU) in a 10 ml vial (type I glass) with a stopper (rubber), a light grey disc (plastic), and a green striped cap (aluminium). 5 ml of solvent in a vial (type I glass) with a stopper (rubber), a disc (plastic), and a cap (aluminium).
AFSTYLA 3000 IU powder and solvent for solution for injection: Powder (3000 IU) in a 10 ml vial (type I glass) with a stopper (rubber), a yellow disc (plastic), and a green striped cap (aluminium). 5 ml of solvent in a vial (type I glass) with a stopper (rubber), a disc (plastic), and a cap (aluminium).
Presentations
One pack with 250, 500 or 1000 IU containing:
1 vial with powder
1 vial with 2.5 ml water for injections
1 filter transfer device 20/20
One inner box containing:
1 disposable 5 ml syringe
1 venipuncture set
2 alcohol swabs
1 non-sterile plaster
One pack with 1500, 2000, 2500 or 3000 IU containing:
1 vial with powder
1 vial with 5 ml water for injections
1 filter transfer device 20/20
One inner box containing:
1 disposable 10 ml syringe
1 venipuncture set
2 alcohol swabs
1 non-sterile plaster
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
General instructions:
The solution should be almost colourless, clear or slightly opalescent. After filtering/withdrawal (see below) the reconstituted product should be inspected visually for particulate matter and discoloration prior to administration.
Do not use visibly cloudy solutions or solutions still containing flakes or particles.
Reconstitution and withdrawal must be carried out under aseptic conditions.
Reconstitution and administration:
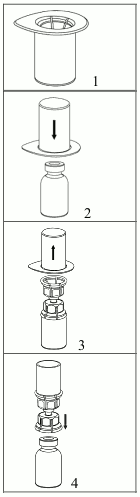
Bring the solvent to room temperature. Ensure powder and solvent vial flip caps are removed and the stoppers are treated with an antiseptic solution and allowed to dry prior to opening the Mix2Vial package.
1. Open the Mix2Vial by peeling off the lid. Do not remove the Mix2Vial from the blister package!
2. Place the solvent vial on an even, clean surface and hold the vial tight. Take the Mix2Vial together with the blister package and push the spike of the blue adapter end straight down through the solvent vial stopper.
3. Carefully remove the blister package from the Mix2Vial set by holding at the rim, and pulling vertically upwards. Make sure that you only pull away the blister package and not the Mix2Vial set.
4. Place the powder vial on an even and firm surface. Invert the solvent vial with the Mix2Vial set attached and push the spike of the transparent adapter end straight down through the powder vial stopper. The solvent will automatically flow into the powder vial.
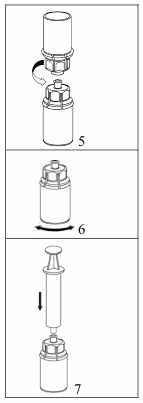
5. With one hand grasp the product-side of the Mix2Vial set and with the other hand grasp the solvent-side and unscrew the set carefully counterclockwise into two pieces. Discard the solvent vial with the blue Mix2Vial adapter attached.
6. Gently swirl the product vial with the transparent adapter attached until the substance is fully dissolved. Do not shake.
7. Draw air into an empty, sterile syringe. While the product vial is upright, connect the syringe to the Mix2Vial's Luer Lock fitting by screwing clockwise. Inject air into the product vial.
Withdrawal and administration:
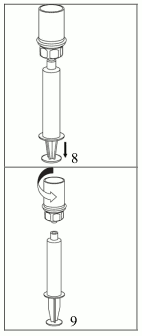
8. While keeping the syringe plunger pressed, turn the system upside down and draw the solution into the syringe by pulling the plunger back slowly.
9. Now that the solution has been transferred into the syringe, firmly hold on to the barrel of the syringe (keeping the syringe plunger facing down) and disconnect the transparent Mix2Vial adapter from the syringe by unscrewing counterclockwise.
For injection of AFSTYLA the provided administration sets are recommended to be used because treatment failure can occur as a consequence of factor VIII adsorption to the internal surface of some injection equipment.
Care should be taken that no blood enters the syringe filled with product, as there is a risk that the blood could coagulate in the syringe and fibrin clots could therefore be administered to the patient.
The AFSTYLA solution must not be diluted.
The reconstituted solution should be administered by a separate injection/infusion line by slow intravenous injection, at a rate comfortable to the patient, up to a maximum of 10 ml/min.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.