ALLUZIENCE Solution for injection Ref.[50428] Active ingredients: Botulinum toxin type A
Source: Health Products Regulatory Authority (IE) Revision Year: 2022 Publisher: Ipsen Pharma, 65 quai Georges Gorse, 92100 Boulogne-Billancourt, France
4.1. Therapeutic indications
Alluzience is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines (vertical lines between the eyebrows) seen at maximum frown in adult patients under 65 years, when the severity of these lines has an important psychological impact on the patient.
4.2. Posology and method of administration
Posology
Botulinum toxin product units differ depending on the medicinal products. Botulinum toxin units are not interchangeable from one product to another. Doses recommended in Speywood units are different from other botulinum toxin preparations.
Paediatric Population
The safety and efficacy of Alluzience in children aged up to 18 years have not been established. The use of Alluzience is not recommended in patients under 18 years.
Method of administration
Alluzience should only be administered by a physician with appropriate qualifications and expertise in this treatment and having the required equipment.
A vial of Alluzience should only be used to treat a single patient, during a single session. Remove any make-up and disinfect the skin with a local antiseptic before administration.
The intramuscular injections should be performed using a sterile needle with a suitable gauge.
Dosing and treatment intervals depend on assessment of the individual patient's response.
The median time to onset as reported subjectively by patients was 3 days (the majority of patients reported an effect within 2 to 3 days with some patients reporting an effect within 24 hours). An effect has been demonstrated for up to 6 months after injection.
The treatment interval should be no more frequent than every 3 months.
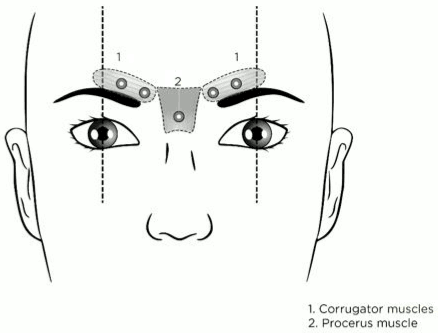
The recommended injection points for glabellar lines are shown below:
Administration instructions
The recommended dose is 0.25 ml of solution (50 Speywood units) divided into 5 injection sites, 0.05 ml of solution (10 Speywood units) administered intramuscularly into each of the 5 sites: 2 injections into each corrugator muscle and one into the procerus muscle, near the nasofrontal angle. The anatomical landmarks can be more readily identified if palpated and observed at patient maximum frown. Before injection, place the thumb or index finger firmly below the orbital rim in order to prevent extravasation below the orbital rim. The needle bevel should be pointed upward and medially during the injection. In order to reduce the risk of ptosis, avoid injections near the levator palpebrae superioris muscle, particularly in patients with larger brow-depressor complexes (depressor supercilii). Injections should be made into the central part of the corrugator muscle, at least 1 cm above the orbital rim.
General information
In the event of treatment failure or diminished effect following repeat injections, alternative treatment methods should be employed. In case of treatment failure after the first treatment session, the following approaches may be considered:
- Analysis of the causes of failure, e.g. incorrect muscles injected, inappropriate injection technique, and formation of toxin-neutralising antibodies
- Re-evaluation of the relevance of treatment with botulinum toxin A.
4.9. Overdose
Excessive doses of botulinum toxin may produce neuromuscular weakness with a variety of symptoms. Respiratory support may be required where excessive doses cause paralysis of respiratory muscles. In the event of overdose, the patient should be medically monitored for symptoms of excessive muscle weakness or muscle paralysis. Symptomatic treatment may be necessary.
Symptoms of overdose may not be present immediately following injection.
Admission to hospital should be considered in patients with symptoms of botulinum toxin overdose (e.g. a combination of muscle weakness, ptosis, diplopia, swallowing and speech disorders, or paresis of the respiratory muscles).
6.3. Shelf life
12 months.
6.4. Special precautions for storage
Store in a refrigerator (2°C-8°C). Do not freeze. Keep vials in the outer carton in order to protect from light.
Once opened, the product should be used immediately.
6.5. Nature and contents of container
Nature of container/closure: Type 1 glass vial, butyl rubber closure and aluminum overseal with a polypropylene flip-off top.
Contents of container: Each vial contains 125 Speywood units of Clostridium botulinum type A toxin-haemagglutinin complex in 0.625 ml of solution. Clear colourless solution.
Pack sizes:
Individual pack size: Pack containing 1 or 2 vials of Alluzience 200 Speywood units/ml solution for injection.
Multiple Pack: A multipack contains 6 individual packs, each including 2 vials of Alluzience 200 Speywood units/ml solution for injection.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Immediately after treatment of the patient, any residual Alluzience which may be present in either vial or syringe should be inactivated with dilute hypochlorite solution (1% available chlorine). Spillage of Alluzience should be wiped up with an absorbent cloth soaked in dilute hypochlorite solution. Any unused product or waste material should be disposed of in accordance with local requirements.
RECOMMENDATIONS SHOULD ANY INCIDENT OCCUR DURING THE HANDLING OF BOTULINUM TOXIN
- Any spills of the product must be wiped up with dry, absorbent material.
- The contaminated surfaces should be cleaned using absorbent material impregnated with a solution of sodium hypochlorite (bleach), then dried.
- If a vial is broken, proceed as mentioned above by carefully collecting the pieces of broken glass and wiping up the product, avoiding any cuts to the skin.
- If the product comes into contact with the skin, wash the affected area with a solution of sodium hypochlorite (bleach) then rinse abundantly with water.
- If product enters into contact with the eyes, rinse thoroughly with plenty of water or with an ophthalmic eyewash solution.
- If product enters into contact with a wound, cut or broken skin, rinse thoroughly with plenty of water and take the appropriate medical steps according to the dose injected.
These instructions for use handling and disposal should be strictly followed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.