ALUNBRIG Film-coated tablet Ref.[10636] Active ingredients: Brigatinib
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Takeda Pharma A/S, Delta Park 45, 2665 Vallensbaek Strand, Denmark
4.1. Therapeutic indications
Alunbrig is indicated as monotherapy for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) previously not treated with an ALK inhibitor.
Alunbrig is indicated as monotherapy for the treatment of adult patients with ALK-positive advanced NSCLC previously treated with crizotinib.
4.2. Posology and method of administration
Treatment with Alunbrig should be initiated and supervised by a physician experienced in the use of anticancer medicinal products.
ALK-positive NSCLC status should be known prior to initiation of Alunbrig therapy. A validated ALK assay is necessary for the selection of ALK-positive NSCLC patients (see section 5.1). Assessment for ALK-positive NSCLC should be performed by laboratories with demonstrated proficiency in the specific technology being utilised.
Posology
The recommended starting dose of Alunbrig is 90 mg once daily for the first 7 days, then 180 mg once daily.
If Alunbrig is interrupted for 14 days or longer for reasons other than adverse reactions, treatment should be resumed at 90 mg once daily for 7 days before increasing to the previously tolerated dose.
If a dose is missed or vomiting occurs after taking a dose, an additional dose should not be administered and the next dose should be taken at the scheduled time. Treatment should continue as long as clinical benefit is observed.
Dose adjustments
Dosing interruption and/or dose reduction may be required based on individual safety and tolerability.
Alunbrig dose reduction levels are summarised in Table 1.
Table 1. Recommended Alunbrig dose reduction levels:
| Dose | Dose reduction levels | ||
|---|---|---|---|
| First | Second | Third | |
| 90 mg once daily (first 7 days) | reduce to 60 mg once daily | permanently discontinue | not applicable |
| 180 mg once daily | reduce to 120 mg once daily | reduce to 90 mg once daily | reduce to 60 mg once daily |
Alunbrig should be permanently discontinued if patient is unable to tolerate the 60 mg once daily dose.
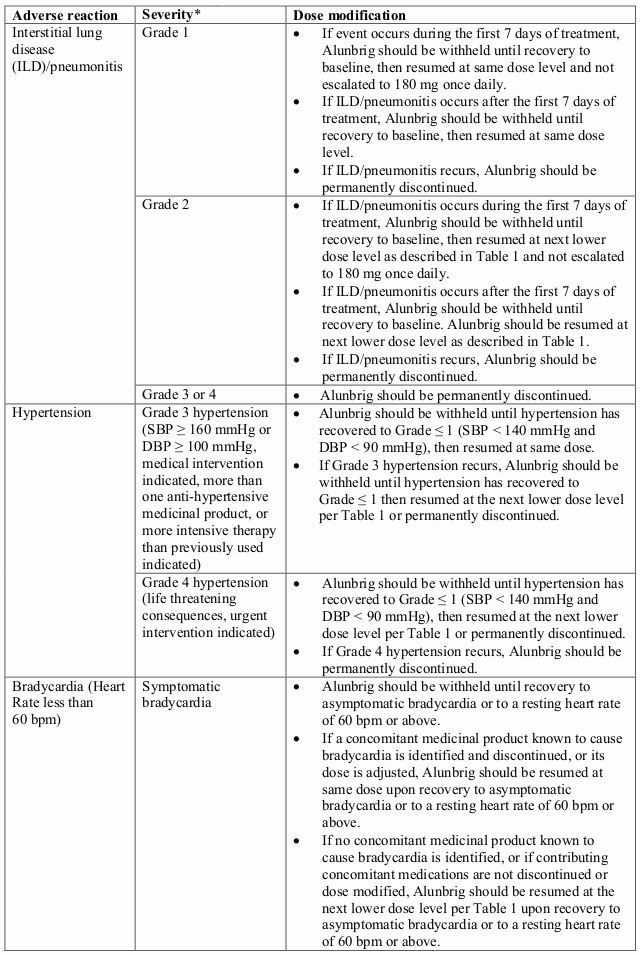
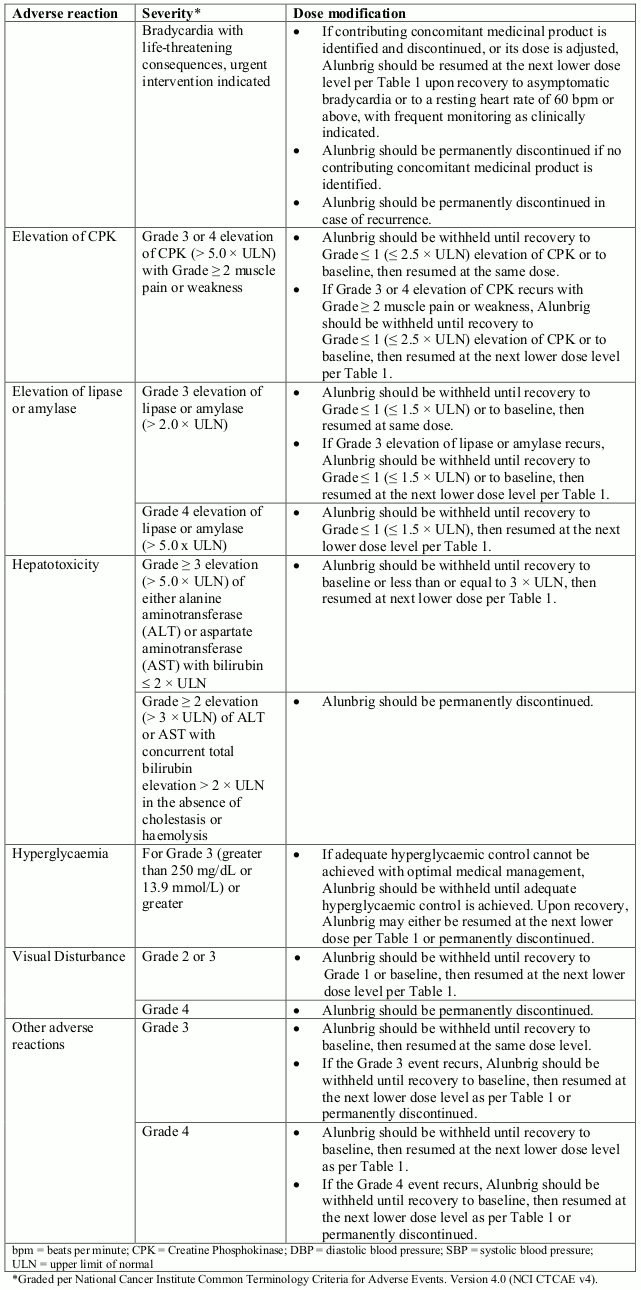
Recommendations for dose modifications of Alunbrig for the management of adverse reactions are summarised in Table 2.
Table 2. Recommended Alunbrig dose modifications for adverse reactions:
Special populations
Elderly patients
The limited data on the safety and efficacy of Alunbrig in patients aged 65 years and older suggest that a dose adjustment is not required in elderly patients (see section 4.8). There are no available data on patients over 85 years of age.
Hepatic impairment
No dose adjustment of Alunbrig is required for patients with mild hepatic impairment (Child-Pugh class A) or moderate hepatic impairment (Child-Pugh class B). A reduced starting dose of 60 mg once daily for the first 7 days, then 120 mg once daily is recommended for patients with severe hepatic impairment (Child-Pugh class C) (see section 5.2).
Renal impairment
No dose adjustment of Alunbrig is required for patients with mild or moderate renal impairment (estimated glomerular filtration rate (eGFR) ≥30 mL/min). A reduced starting dose of 60 mg once daily for the first 7 days, then 90 mg once daily is recommended for patients with severe renal impairment (eGFR <30 mL/min) (see section 5.2). Patients with severe renal impairment should be closely monitored for new or worsening respiratory symptoms that may indicate ILD/pneumonitis (e.g., dyspnoea, cough, etc.) particularly in the first week (see section 4.4).
Paediatric population
The safety and efficacy of Alunbrig in patients less than 18 years of age have not been established. No data are available.
Method of administration
Alunbrig is for oral use. The tablets should be swallowed whole and with water. Alunbrig may be taken with or without food.
Grapefruit or grapefruit juice may increase plasma concentrations of brigatinib and should be avoided (see section 4.5).
4.9. Overdose
There is no specific antidote for overdose with Alunbrig. In the event of an overdose, monitor the patient for adverse reactions (see section 4.8) and provide appropriate supportive care.
6.3. Shelf life
3 years.
6.4. Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5. Nature and contents of container
Alunbrig 30 mg film-coated tablets:
Round wide mouth high density polyethylene (HDPE) bottles with two-piece polypropylene child resistant screw cap closures with foil induction seal liner, containing either 60 or 120 film-coated tablets, together with one HDPE canister containing a molecular sieve desiccant.
Clear thermoformable poly-chloro-tri-fluoro-ethylene (PCTFE) blister with heat sealable paper-laminated foil lidding in a carton, containing either 28, 56 or 112 film-coated tablets.
Alunbrig 90 mg film-coated tablets:
Round wide mouth high density polyethylene (HDPE) bottles with two-piece polypropylene child resistant screw cap with foil induction seal liner closures, containing either 7 or 30 film-coated tablets, together with one HDPE canister containing a molecular sieve desiccant.
Clear thermoformable poly-chloro-tri-fluoro-ethylene (PCTFE) blister with heat sealable paper-laminated foil lidding in a carton, containing either 7 or 28 film-coated tablets.
Alunbrig 180 mg film-coated tablets:
Round wide mouth high density polyethylene (HDPE) bottles with two-piece polypropylene child resistant screw cap with foil induction seal liner closures, containing 30 film-coated tablets, together with one HDPE canister containing a molecular sieve desiccant.
Clear thermoformable poly-chloro-tri-fluoro-ethylene (PCTFE) blister with heat sealable paper-laminated foil lidding in a carton, containing 28 film-coated tablets.
Treatment initiation pack Alunbrig 90 mg and 180 mg film-coated tablets:
Each pack consists of an outer carton with two inner cartons containing:
- Alunbrig 90 mg film-coated tablets 1 clear thermoformable poly-chloro-tri-fluoro-ethylene (PCTFE) blister with heat sealable paper-laminated foil lidding in a carton, containing 7 film-coated tablets.
- Alunbrig 180 mg film-coated tablets 3 clear thermoformable poly-chloro-tri-fluoro-ethylene (PCTFE) blisters with heat sealable paper-laminated foil lidding in a carton, containing 21 film-coated tablets.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Patients should be advised to keep the desiccant canister in the bottle and not to swallow it.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.