ANDRODERM Transdermal system Ref.[10747] Active ingredients: Testosterone
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis and scrotum; the development of male hair distribution, such as facial, pubic, chest and axillary hair; laryngeal enlargement; vocal cord thickening; and alterations in body musculature and fat distribution. Testosterone and DHT are necessary for the normal development of secondary sex characteristics.
Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter Syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH).
12.2. Pharmacodynamics
No specific pharmacodynamic studies were conducted using ANDRODERM.
12.3. Pharmacokinetics
Absorption
ANDRODERM delivers physiologic amounts of testosterone, producing circulating testosterone concentrations that approximate the normal concentration range (300-1030 ng/dL) seen in healthy men. ANDRODERM provides a continuous daily dose of testosterone in a self-contained transdermal system. Following ANDRODERM application, testosterone is continuously absorbed during the 24-hour dosing period with a median (range) Tmax of 8 (4-12) hours.
Distribution
Circulating testosterone is primarily bound in the serum to sex hormone-binding globulin (SHBG) and albumin. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is bound to albumin and other proteins.
Metabolism
Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are estradiol and dihydrotestosterone (DHT).
During steady-state pharmacokinetic studies in hypogonadal men treated with ANDRODERM, the average DHT:T and E2:T ratios were approximately 1:10 and 1:200, respectively.
Excretion
There is considerable variation in the half-life of testosterone as reported in the literature, ranging from 10 to 100 minutes. About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver.
Upon removal of the ANDRODERM systems, serum testosterone concentrations decrease with an apparent half-life of approximately 70 minutes. Hypogonadal concentrations are reached within 24 hours following system removal. There is no accumulation of testosterone during continuous treatment.
Effect of Showering
In a two-way crossover study, the effects of showering on the pharmacokinetics of total testosterone following a single application of ANDRODERM 4 mg/day were assessed in 16 hypogonadal males. Showering 3 hours after application of ANDRODERM increased Cavg by 0.5% and decreased Cmax by 0.4% respectively, as compared to not showering. The systemic exposure to ANDRODERM was similar following applications with or without showering 3 hours after application.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant induced cervical-uterine tumors, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Mutagenesis
Testosterone was negative in the in vitro Ames and in the in vivo mouse micronucleus assays.
Impairment of Fertility
The administration of exogenous testosterone has been reported to suppress spermatogenesis in the rat, dog and non-human primates, which was reversible on cessation of the treatment.
14. Clinical Studies
ANDRODERM 2 mg/day and 4 mg/day were studied in a trial designed to evaluate the use and titration of 2 mg/day and 4 mg/day systems in a clinic setting of 40 men with hypogonadism. Thirty-eight of the 40 subjects (95%) who were enrolled into the study were white and 2 subjects were African American. Ten (25%) subjects were Hispanic and 30 (75%) were Non-Hispanic. Men were between 34 and 76 years of age (mean: 55 years). Patients had previously been on stable therapy of ANDRODERM 5 mg; Androgel 2.5 grams, 5 grams, 7.5 grams or 10 grams; or Testim 2.5 grams or 5 grams daily before switching to ANDRODERM 4 mg/day.
Patients applied an ANDRODERM 4 mg/day system around 10 p.m. once daily for 14 days, and then were titrated up to 6 mg/day or down to 2 mg/day according to a morning serum testosterone concentration obtained at 6 a.m. on Day 8. Out of 36 patients who entered the study, 31 (86%) patients remained on the 4 mg/day dose, 4 (11%) were titrated downward to 2 mg/day, and 1 (3%) was titrated upward to 6 mg/day based on the Day 8 testosterone concentrations. The one patient that was titrated to 6 mg/day discontinued from the study for a non-safety related reason. Of the patients who were receiving ANDRODERM 5 mg/day prior to study entry (n=11), 10 remained at 4 mg/day after titration, and 1 was titrated down to the 2 mg/day dose.
After a total of 28 days of therapy, 34 of the 35 subjects (97%) had serum testosterone Cavg within the normal range during the dosing period, with the lower bound of the 95% confidence interval for this estimate being 85% (Table 3). One subject who received ANDRODERM 4 mg/day treatment had serum testosterone Cavg below 300 ng/dL and none had Cavg concentrations above 1030 ng/dL. The mean (SD) serum testosterone Cmax following treatment with the 2 mg/day (N=4) and 4 mg/day (N=31) systems was 648 (145) ng/dL and 696 (158) ng/dL, respectively. Table 3 summarizes testosterone Cavg categories by treatment.
Table 3. Testosterone Cavg Categories on Day 28 after One Titration on Day 15:
| Cavg Category | Current Testosterone User N=35 |
|---|---|
| 300-1030 ng/dL (n (%) (95% CI)) | 34/35 (97%) (85%, 100%) |
| <300 ng/dL (n (%)) | 1/35 (3%) |
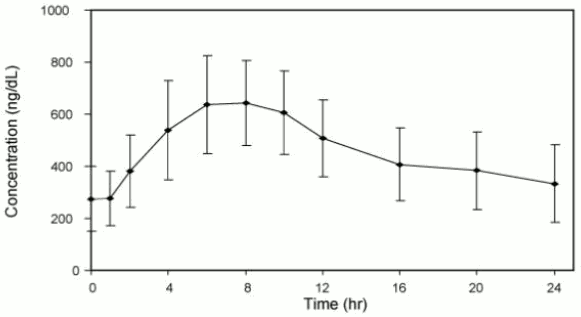
Figure 2 summarizes the pharmacokinetic profiles of total testosterone in 35 patients completing 28 days of ANDRODERM treatment applied as a starting dose of 4 mg/day for the initial 14 days followed by a possible dose titration.
Figure 2. Mean (SD) Steady-State Serum Total Testosterone Concentration (ng/dL) on Day 28:
In separate clinical studies using the ANDRODERM 2.5 mg/day system, 1% used 2.5 mg daily, 93% of patients used 5 mg daily, and 6% used 7.5 mg daily. The hormonal effects of ANDRODERM 2.5 mg/day system as a treatment for male hypogonadism was demonstrated in four open-label trials that included 94 hypogonadal men, ages 15 to 65 years. In these trials, ANDRODERM produced average morning serum testosterone concentrations within the normal reference range in 92% of patients.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.