ANDROXY Tablet Ref.[10086] Active ingredients: Fluoxymesterone
Source: FDA, National Drug Code (US) Revision Year: 2017
Product description
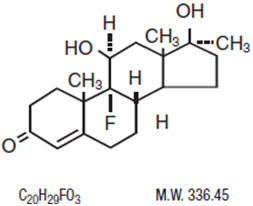
ANDROXY (Fluoxymesterone Tablets, USP) contains fluoxymesterone, a synthetic androgen. The androgens are steroids that develop and maintain primary and secondary male sex characteristics. Androgens are derivatives of cyclopentano-perhydrophenanthrene. Endogenous androgens are C-19 steroids with a side chain at C-17, and with two angular methyl groups. Testosterone is the primary endogenous androgen. Fluoxymesterone is a synthetic derivative of testosterone.
In their active form, all drugs in the class have a 17-beta-hydroxy group. 17-alpha-alkylation and halogenation at position 9 (fluoxymesterone) increase the pharmacologic activity per unit weight compared to testosterone when given orally. Fluoxymesterone is a white or practically white odorless, crystalline powder, melting at about 240°C with some decomposition. It is practically insoluble in water, sparingly soluble in alcohol and slightly soluble in chloroform. Chemically fluoxymesterone is designated 9-fluoro-11β, 17β-dihydroxy-17-methylandrost-4-en-3-one. Structurally it may be represented as follows:
ANDROXY (Fluoxymesterone Tablets, USP) for oral administration contains 10 mg of fluoxymesterone USP.
Inactive Ingredients: croscarmellose sodium, D&C Yellow #10 Aluminum Lake, FD&C Blue #1 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, anhydrous lactose, magnesium stearate, microcrystalline cellulose, pregelatinized starch (corn), and sodium lauryl sulfate.
| How Supplied |
|---|
|
ANDROXY (Fluoxymesterone Tablets, USP) 10 mg are round, green, scored compressed tablets debossed with 832 and 86 and are available in bottles of 100. Dispense in a tight, light-resistant container as defined in the USP. Manufactured by UPSHER-SMITH LABORATORIES, LLC, Maple Grove, MN 55369 |
Drugs
| Drug | Countries | |
|---|---|---|
| ANDROXY | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.