ANTABUSE Tablet Ref.[27450] Active ingredients: Disulfiram
Source: FDA, National Drug Code (US) Revision Year: 2015
Product description
Disulfiram, USP is an alcohol antagonist drug.
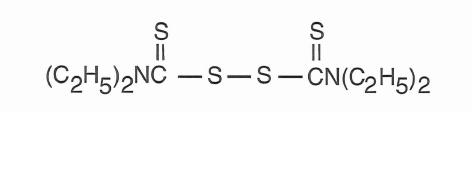
Chemical name:
bis(diethylthiocarbamoyl) disulfide.
Structural formula:
C10H20N2S4 M.W. 296.54
Disulfiram, USP occurs as a white to off-white, odorless, and almost tasteless powder, soluble in water to the extent of about 20 mg in 100 mL, and in alcohol to the extent of about 3.8 g in 100 mL.
Each tablet for oral administration contains 250 mg or 500 mg disulfiram, USP. Tablets also contain colloidal silicon dioxide, lactose anhydrous, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and stearic acid.
| How Supplied |
|---|
|
Disulfiram Tablets USP are available as follows: 250 mg – white, round, unscored, biconvex tablets, debossed with OP over 706 on one side and plain on the other side, in bottles of 100 tablets (NDC 51285-523-02). 500 mg – white, round, scored tablets, debossed with OP over 707 on one side and scored on the other side, in bottles of 100 tablets (NDC 51285-524-02). Manufactured In Croatia By: PLIVA HRVATSKA d.o.o., Zagreb, Croatia Manufactured For: TEVA PHARMACEUTICALS USA, INC., North Wales, PA 19454 |
Drugs
| Drug | Countries | |
|---|---|---|
| ANTABUSE | Austria, Ireland, New Zealand, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.